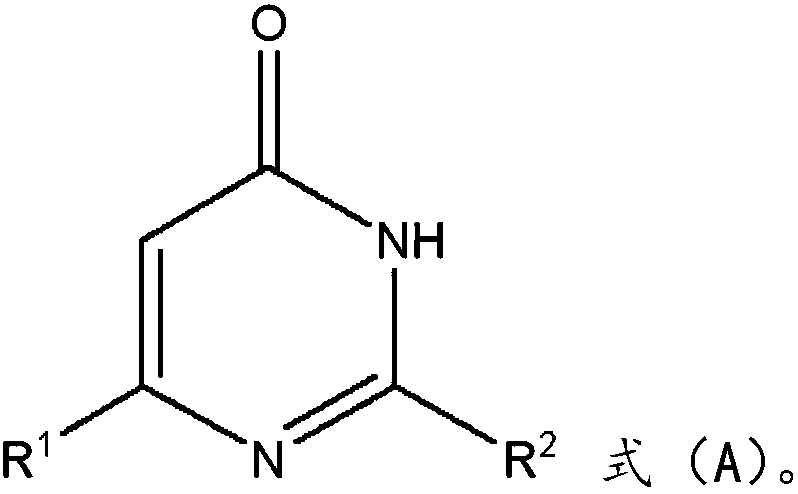
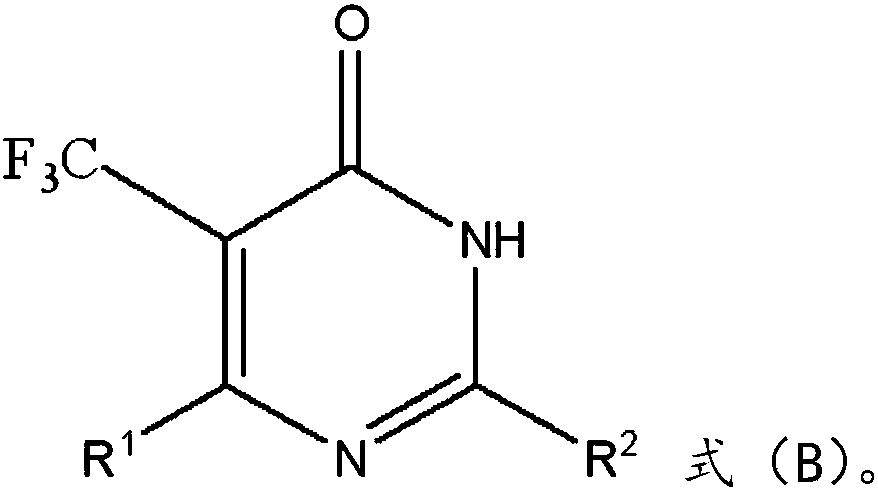
Method for synthesizing fluorine-containing pyrimidinone compound
A technology for fluorine-containing pyrimidone and a synthesis method, which is applied in the field of synthesis of fluorine-containing pyrimidone compounds, can solve the problems of high cost, unfriendly environment, limited synthesis method, etc., and achieves low cost, environment-friendly and simple reaction system. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The embodiment of the present invention provides a method for synthesizing fluoropyrimidinone compounds, comprising the following steps:
[0022] Pyrimidinone compounds and sodium trifluoromethanesulfinate are respectively added into the reaction bottle, and reacted at 25° C.-80° C. for 2-4 hours under the action of tert-butyl perbenzoate and water.
[0023] In this step, pyrimidinone compounds and sodium trifluoromethylsulfinate are used to synthesize fluorine-containing pyrimidinone compounds. In this step, sodium trifluoromethylsulfinate generates trifluoromethyl cations under the action of TBPB, and trifluoromethyl cations electrophilically attack pyrimidinone compounds, and then leave hydrogen ions to generate target products. It should also be noted here that this step does not require the participation of an organic solvent, and only water needs to be used as a solvent, which is not only environmentally friendly, but also can effectively increase the product yiel...
Embodiment 1
[0040] Add 1mmol of pyrimidinone compound, 1mmol of sodium trifluoromethyl sulfinate, 2mmol of tert-butyl perbenzoate and 1mL of water into a reaction tube, and react at 25°C for 2 hours. After the reaction is completed, the column chromatographic separation yields the following target: Compound:
[0041] Carry out nuclear magnetic spectrum analysis to above-mentioned white crystal powder, data is as follows:
[0042] 1 H NMR (DMSO-d6, 400MHz): δ13.64(s, 1H), 8.18(d, J=7.5Hz, 2H), 7.69(t, J=7.3Hz, 1H), 7.49-7.61(m, 7H );
[0043] 13 C NMR (DMSO-d6, 100MHz): d 165.5, 160.9, 159.8, 138.8, 133.3, 131.8, 130.1, 129.1, 129.0, 128.60, 128.57, 128.3, 123.9, 110.9;
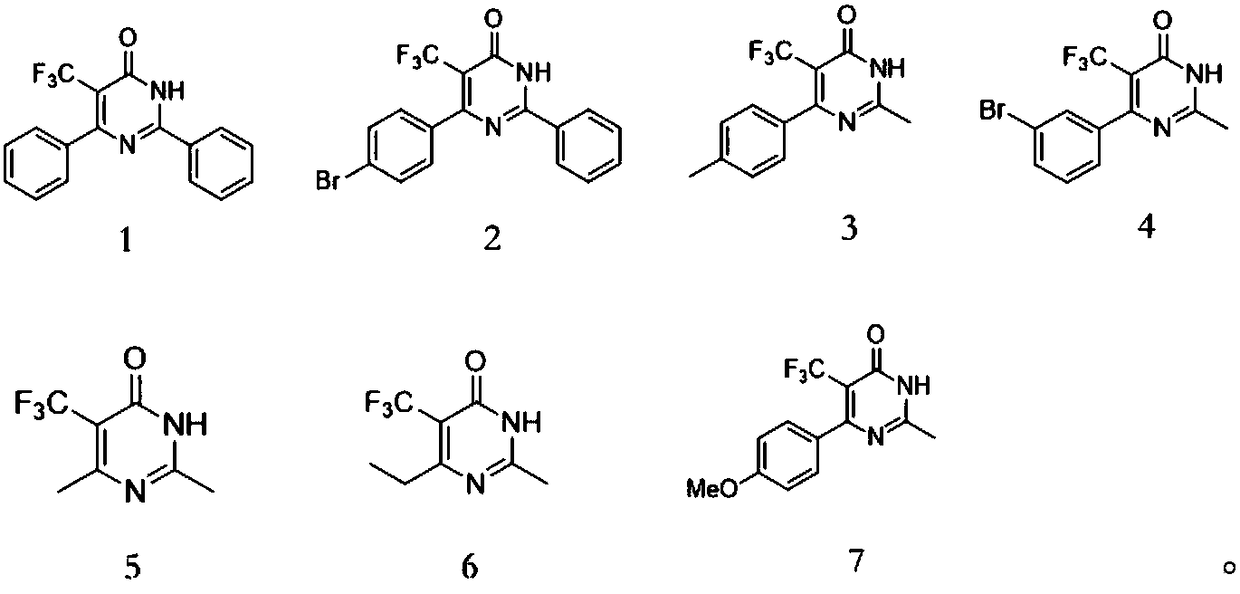
[0044] After identification, the spectral data corresponded to the structural formula, proving that the synthesized product was 2,6-diphenyl-5-(trifluoromethyl)pyrimidin-4(3H)-one with a yield of 96%.
Embodiment 2
[0046] Add 1mmol of pyrimidinone compound, 1mmol of sodium trifluoromethyl sulfinate, 2mmol of tert-butyl perbenzoate and 1mL of water into a reaction tube, react at 65°C for 4 hours, and separate by column chromatography after the reaction, the following target is obtained Compound:
[0047] Carry out nuclear magnetic spectrum analysis to above-mentioned white crystal powder, data is as follows:
[0048] 1 H NMR (DMSO-d6, 400MHz): δ13.59(s, 1H), 8.25(d, J=7.0Hz, 2H), 7.74(d, J=7.7Hz, 2H), 7.68-7.40(m, 5H );
[0049] 13 C NMR (DMSO-d6, 100MHz): d 164.3, 160.9, 158.0, 137.6, 133.4, 132.0, 131.8, 130.9, 129.1, 129.0, 123.8, 123.8;
[0050] After identification, the spectral data corresponded to the structural formula, proving that the synthesized product was 6-(4-bromophenyl)-2-phenyl-5-(trifluoromethyl)pyrimidin-4(3H)-one, and the yield was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com