Catalyst for silylation of aromatic amines
A technology for silylation and aromatic amines, which is applied in the field of catalysts for silylation of aromatic amines, can solve the problems of high toxicity, high price, and reduced use performance and safety of silylated aromatic amines, and achieves improved yield , low price, avoid the effect of device performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment provides a kind of Al(C 6 f 5 ) 3 The preparation method of catalyst specifically comprises the following steps:
[0047] (1) In the glove box, 3.00 g of B(C 6 f 5 ) 3 Add to the mixed solvent of 10mL toluene and 30mL hexene, take 3.00mL of Al(CH 3 ) 3 solution, Al(CH 3 ) 3 The solution is a 2.0M molarity solution in hexane. Al(CH 3 ) 3 The solution was added to the reactor via a syringe, and B(C 6 f 5 ) 3 The solutions were mixed to obtain a mixture.
[0048] (2) The mixture started to precipitate after stirring at room temperature for 5 minutes, resulting in a crystalline solid. The mixture was stirred at room temperature for 2 h, then filtered.
[0049] (3) The collected crystalline solid was washed with hexene and dried in vacuo to obtain 1.98 g of the product. Slow cooling of the filtrate at -35°C yielded an additional 1.20 g of product, which was washed with hexane and dried in vacuo to afford Al(C 6 f 5 ) 3 The total product is...
Embodiment 2
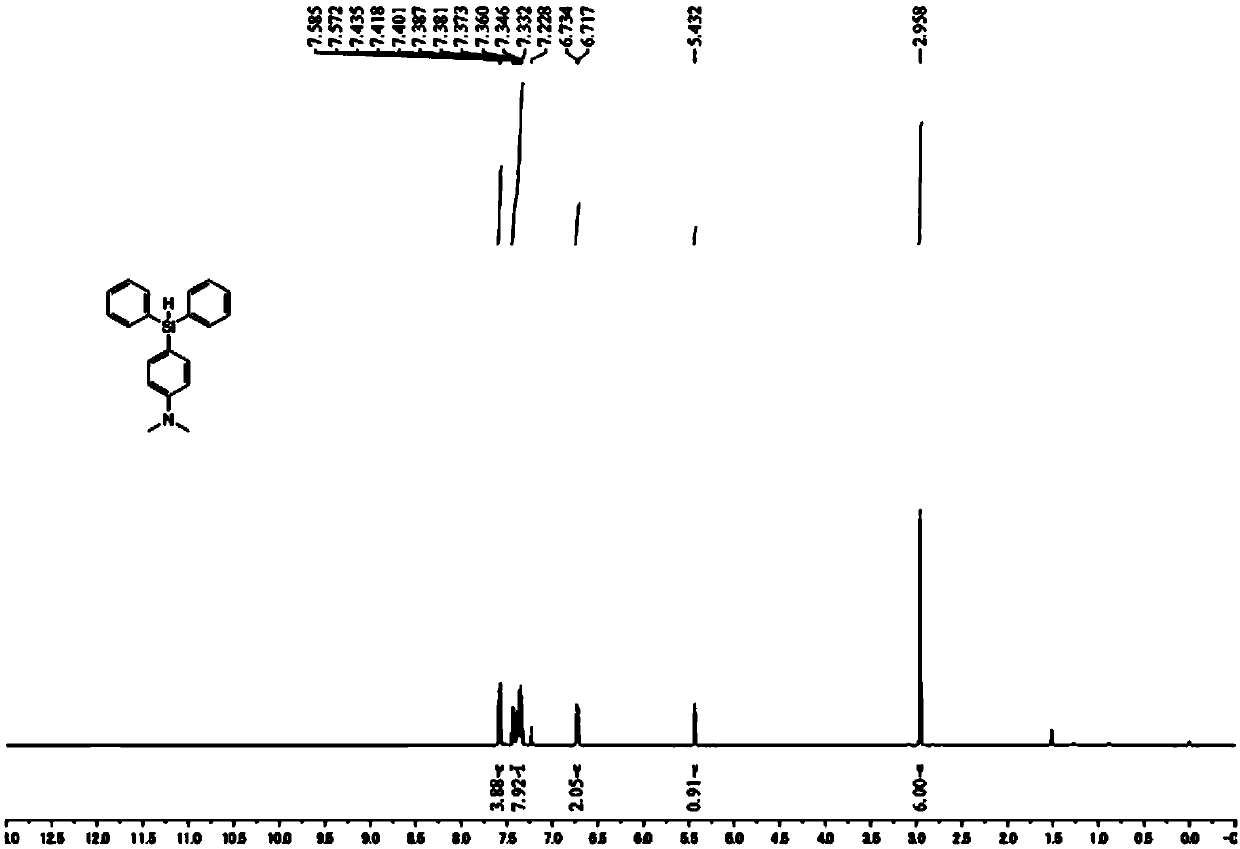
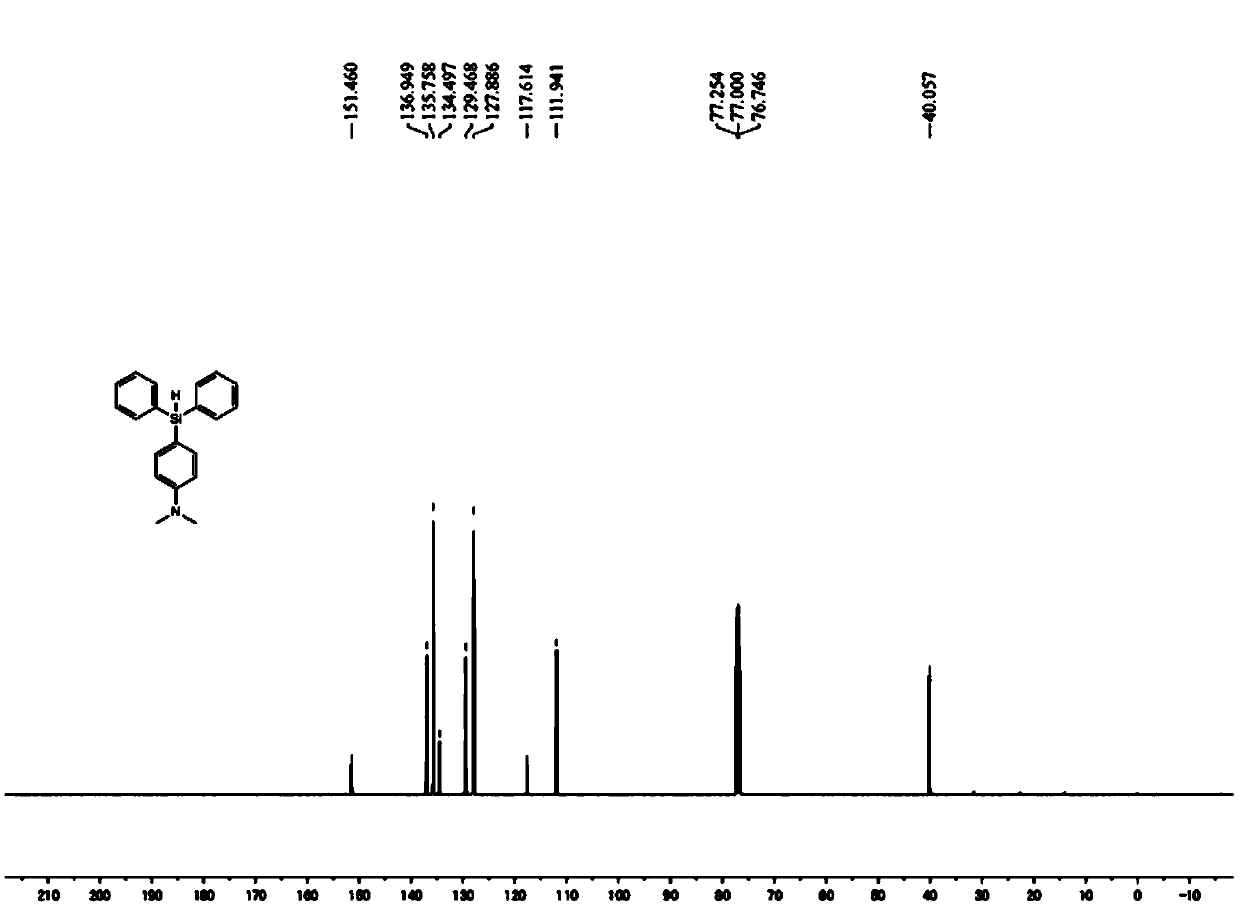
[0051] This embodiment provides a method for preparing a silylated aromatic amine, wherein the silylated aromatic amine is (4-diphenylsilyl-phenyl)-dimethyl-amine, and the reaction process uses the method prepared in Example 1. Al(C 6 f 5 ) 3 As a catalyst, the reaction scheme for the preparation of silylated aromatic amines is shown below:
[0052]
[0053] The preparation method of (4-diphenylsilyl-phenyl)-dimethyl-amine comprises the following steps:
[0054] (1) In the glove box, put Al(C 6 f 5 ) 3 (10.6mg, 0.02mmol) and 2mL of benzene were mixed and added to a 20mL Shrek reaction tube (Schlenk tube); then the aromatic amine (N,N-dimethyl Aniline, 24.2mg, 0.20mmol) and hydrosilane (Ph 2 SiH 2 , 73.6mg, 0.40mmol).
[0055] (2) Take out the Schlenk tube, and stir magnetically for 6 hours in an oil bath environment at 100°C.
[0056] (3) After the reaction is completed, cool the mixed solution in the Schlenk tube to room temperature, dilute the mixed solution wit...
Embodiment 3
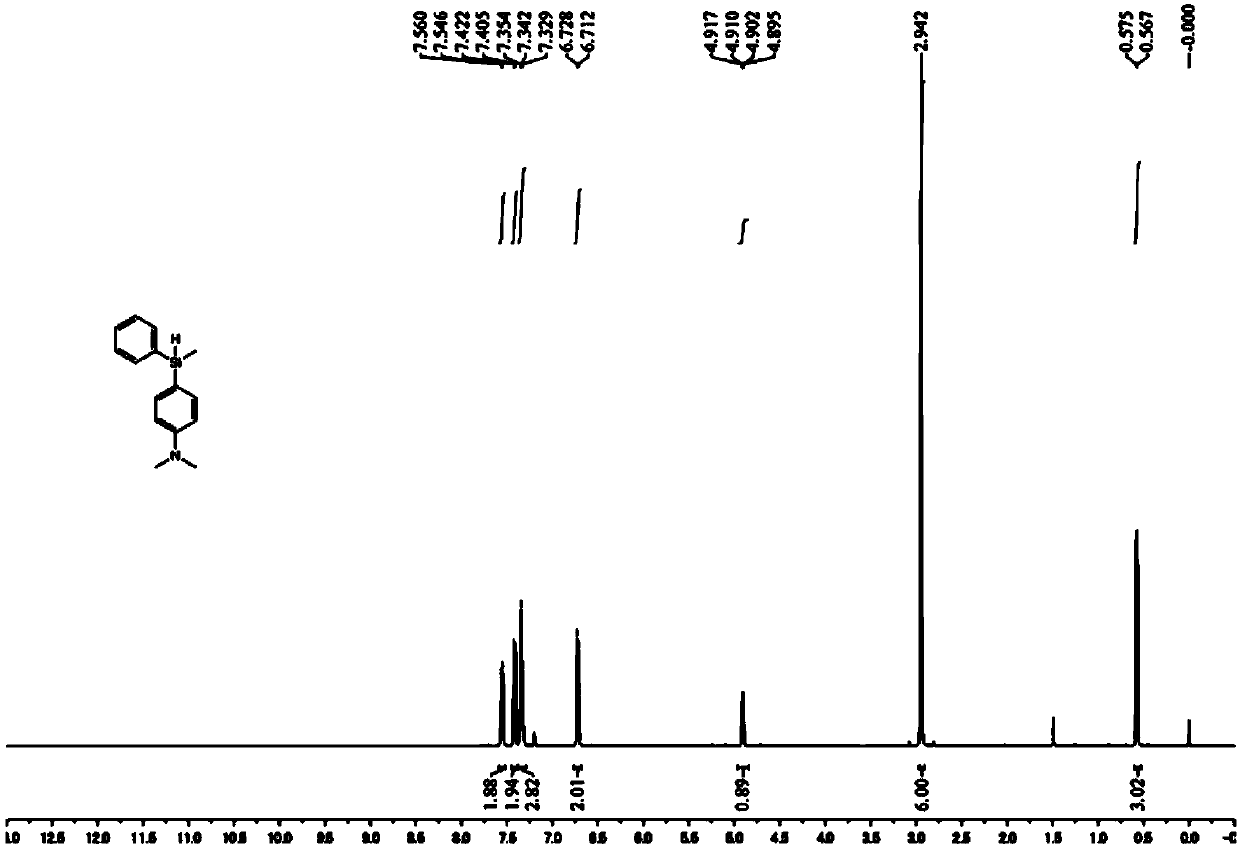
[0060] This embodiment provides a method for preparing a silylated aromatic amine, wherein the silylated aromatic amine is dimethyl-[4-(methyl-phenyl-silyl)phenyl]-amine, and the reaction process is implemented using The Al(C prepared by Example 1 6 f 5 ) 3 As a catalyst, the reaction scheme for the preparation of silylated aromatic amines is shown below:
[0061]
[0062] The preparation method of dimethyl-[4-(methyl-phenyl-silyl) phenyl]-amine comprises the following steps:
[0063] (1) In the glove box, put Al(C 6 f 5 ) 3 (21.2mg, 0.04mmol) and 2mL of toluene were mixed and added to a 20mL Shrek reaction tube (Schlenk tube); then the aromatic amine (N,N-dimethyl Aniline, 24.2mg, 0.20mmol) and hydrosilane represented by formula (II-2) (97.6mg, 0.80mmol).
[0064] (2) Take out the Schlenk tube, and stir magnetically for 6 hours in an oil bath environment at 80°C.
[0065] (3) After the reaction is completed, cool the mixed solution in the Schlenk tube to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com