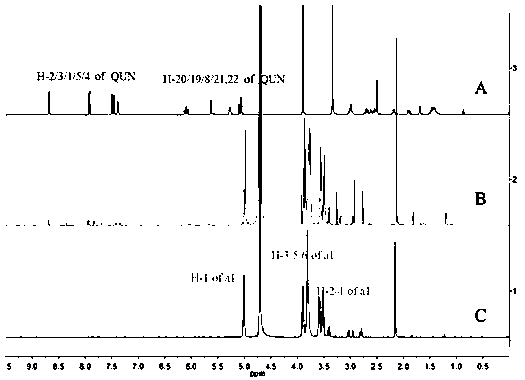
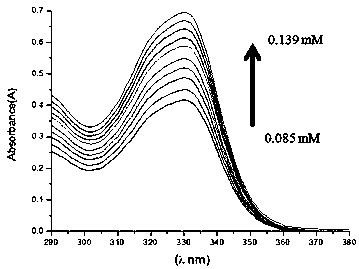
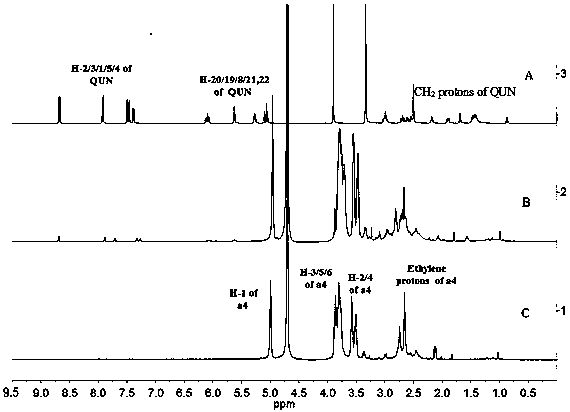
Inclusion compound of quinidine and amine cyclodextrin
A technology of cyclodextrin and inclusion complex, which is applied in the directions of active ingredients of heterocyclic compounds, pharmaceutical combinations, and medical preparations of non-active ingredients, etc. The requirements of drug application, etc., to achieve the effects of easy operation, mild conditions, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Inclusion Complex of Quinidine and Mono-(6-amino-6-deoxy)-β-cyclodextrin
[0037] (1) Preparation of mono-6-OTs-β-CD
[0038] Take 210 g of β-cyclodextrin recrystallized with water, add it in batches to a three-necked flask (3 L) filled with 1300 mL of water, and stir at room temperature; weigh 17.2 g of sodium hydroxide and dissolve it in 50 mL of water, then slowly Add it dropwise to the suspension of β-cyclodextrin until the reaction solution gradually becomes clear, and continue stirring for 1.5 h; after the cyclodextrin in the reaction solution is completely dissolved and becomes clear, dissolve 26.0 g of p-toluenesulfonyl chloride in 80 mL Sonicate the acetonitrile until it becomes clear, and slowly add the solution dropwise to the reaction solution (dropping is completed in about 30 minutes), continue to stir for 2 hours, filter off the insoluble matter, and adjust the filtrate to pH = 7.5 with 2 mol / L hydrochloric acid. At this time, there is a la...
Embodiment 2
[0047] Preparation of Inclusion Complex of Quinidine and Mono-(6-Ethylenediamine-6-deoxy)-β-cyclodextrin
[0048] (1) The preparation method of mono-6-OTs-β-CD is the same as step (1) in Example 1.
[0049] (2) Preparation of mono-(6-ethylenediamine-6-deoxy)-β-cyclodextrin
[0050] Dissolve 3.0 g (2.3 mmol) of 6-OTs-βCD in 20 mL of molecular sieve-dried ethylenediamine, stir at room temperature until completely dissolved, react for 10 h under nitrogen protection at 80 °C, and evaporate under reduced pressure after the reaction is complete. Solvent, add a small amount of water to the reaction flask to dissolve the solid, continue to evaporate the solvent, then add 2 mL of water to dissolve the product, and slowly drop it into 400 mL of acetone, stir at room temperature for 30 min, collect the white precipitate by suction filtration, and The resulting crude solid was dried in a vacuum oven for 24 h. The dried solid powder was redissolved in 2 mL of distilled water, filtered to...
Embodiment 3
[0054] Preparation of Inclusion Complex of Quinidine and Mono-(6-diethylenetriamine-6-deoxy)-β-cyclodextrin
[0055] (1) The preparation method of mono-6-OTs-β-CD is the same as step (1) in Example 1.
[0056] (2) Preparation of mono-(6-diethylenetriamine-6-deoxy)-β-cyclodextrin
[0057] Dissolve 3.0 g (2.3 mmol) of mono-6-OTs-β-CD in 20 mL of dry diethylenetriamine, stir at room temperature until completely dissolved, and react at 80°C for 10 h under nitrogen protection. After the reaction is completed, Slowly drop the reaction solution into 400 mL of acetone, stir at room temperature for 30 min, remove the filtrate by suction filtration, collect the white precipitate, dissolve the precipitate in a small amount of water, drop it into 300 mL of acetone again, stir for 0.5 h, and then suction filter The white precipitate was collected and dried in a vacuum oven. The obtained solid powder was ground and added to 200 mL of ethanol, and the white precipitate was collected by ultr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com