Magnetic particle-based hydrocortisone chemiluminescence immune assay method and kit
A chemiluminescence immunoassay, hydrocortisone technology, applied in chemical instruments and methods, chemiluminescence/bioluminescence, analysis by chemical reaction of materials, etc. Affect the accuracy and accuracy of experimental results, and achieve the effects of accurate detection results, stable substrates and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
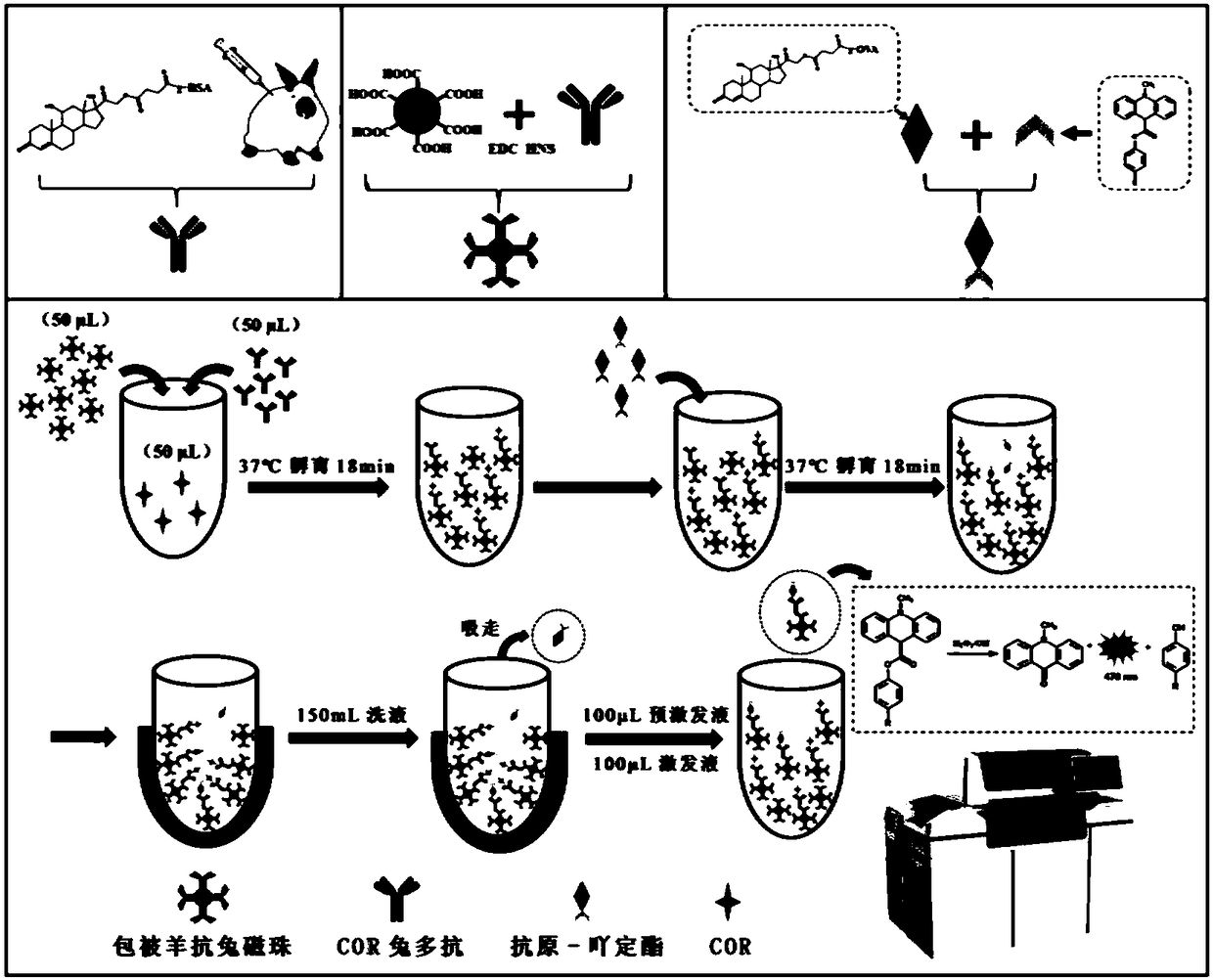
[0054] Example 1 Construction of magnetic particle chemiluminescence immunoassay method
[0055] 1. Preparation of rabbit-derived polyclonal antibody against hydrocortisone
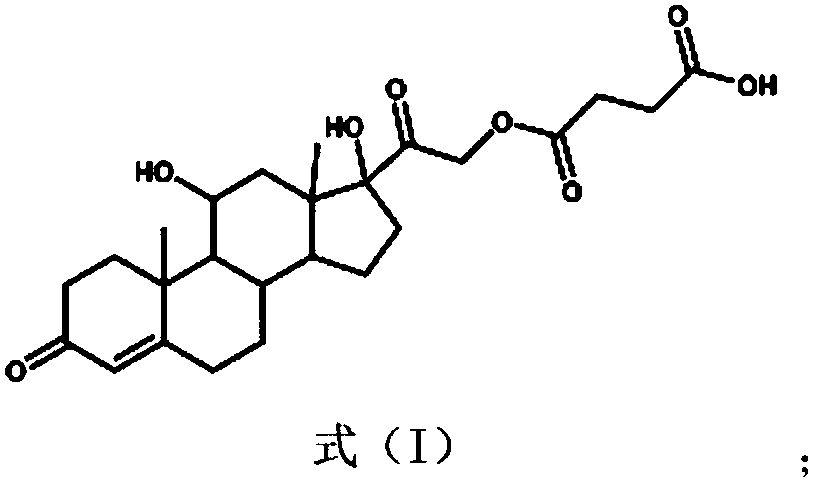
[0056] (1) Antigen preparation: First, take 600mg hydrocortisone in 5mL N,N-dimethylformamide (DMF), add 44.2mg 4-dimethylaminopyridine (DMAP) and 163.8mg succinic anhydride and stir React for 24 hours, spin dry, dissolve the colloidal substance in chloroform, add 20mL distilled water and stir to obtain a large amount of light yellow crystals, carry out suction filtration, rinse the filter residue with excess distilled water and dry it, place the filter residue in 100mL saturated sodium bicarbonate solution, mix After uniformity, centrifuge to take the supernatant, acidify the supernatant with 1mol / L hydrochloric acid to pH 4, filter the filter residue obtained by suction, which is the hapten, and purify it through the column; take 10mg of the hapten and dissolve it in 500μL of anhydrous DMF, add 5.3mg N...
Embodiment 2
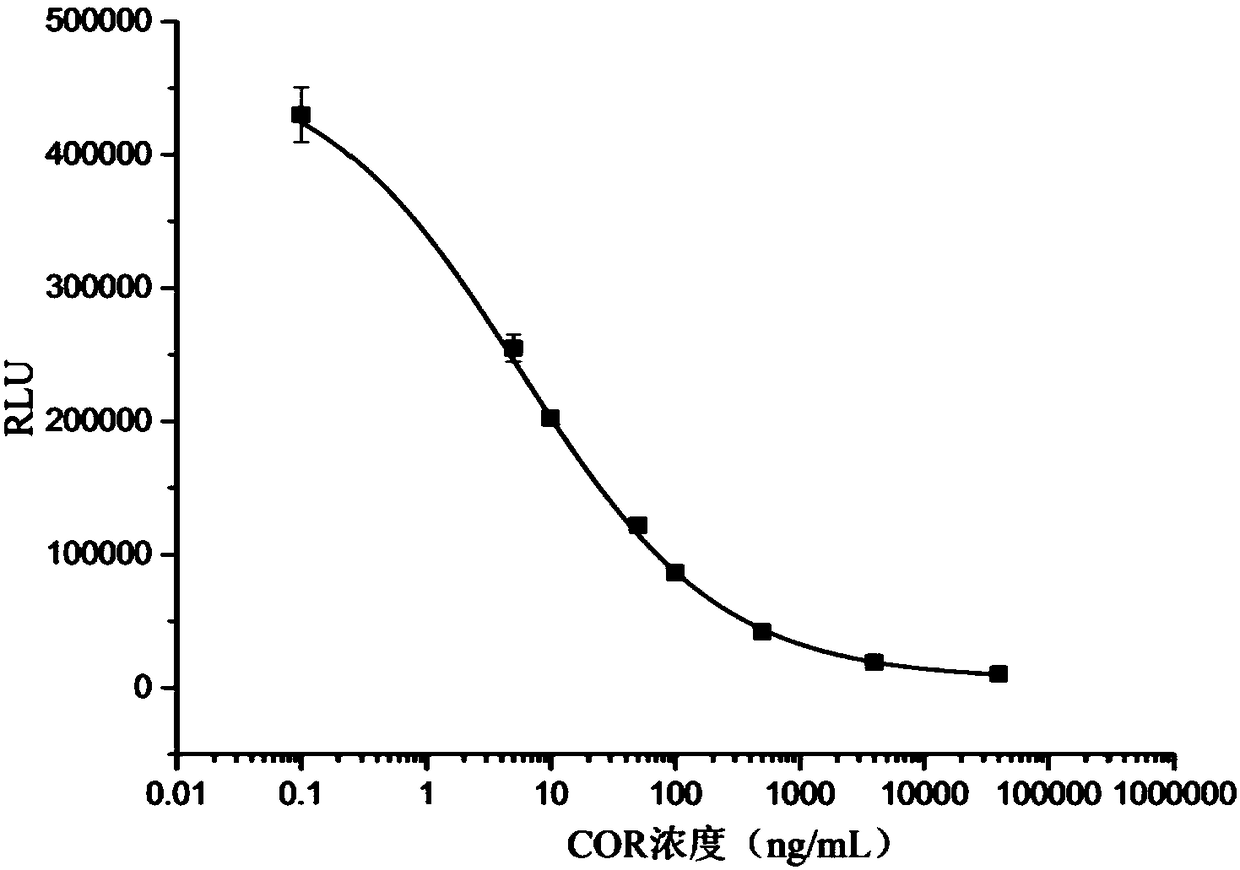
[0070] The establishment of embodiment 2 standard curve
[0071] 1. According to the optimal value determined by the optimization of the above conditions, the luminescence of a series of standard solutions with a solubility of 0.1, 5, 10, 50, 100, 500, 4000 and 40000 ng / mL prepared with 0.01 mol / L PBS solution respectively The value (RLU) is the ordinate, the logarithm of the standard concentration of hydrocortisone is the abscissa, and the standard curve is drawn using Origin9.0 software.
[0072] 2. The detection linear range (IC) of the standard curve based on magnetic particle chemiluminescence immunoassay 20 ~IC 80 ) is 0.42~72.27ng / mL, 50% competitive inhibition (IC 50 ) was 5.57ng / mL, the limit of detection (IC 10 ) is 0.12 ng / mL.
Embodiment 3
[0073] Example 3 Stability Evaluation Based on Magnetic Particle Chemiluminescence Immunoassay Method
[0074] 1. In this study, the stability of the method was evaluated by performing 5 measurements of hydrocortisone solutions with different concentrations of 0.1, 5, 10, 50, 100, 500, 4000 and 40000 ng / mL.
[0075] 2. The experimental results show that the coefficients of variation obtained by measuring 5 times of hydrocortisone solutions with concentrations of 0.1, 5, 10, 50, 100, 500, 4000 and 40000 ng / mL are 3.3%, 3.3%, and 2.1%, respectively. , 5.2%, 2.2%, 7.0%, 7.5% and 5.1%, indicating that the method has good reproducibility.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com