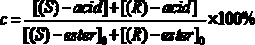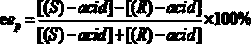Method for stereoselective enzymatic hydrolysis to resolve 2-(3-chlorophenyl)propionic acid enantiomers
A stereoselective, catalytic hydrolysis technology, applied in the direction of fermentation, etc., can solve the problems of low conversion rate and low solubility, and achieve the effects of improving conversion rate, mild reaction conditions and overcoming toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
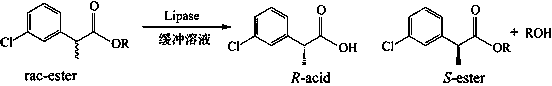
[0026] Put 0.020 mmol of racemic heptyl 2-(3-chlorophenyl)propionate in a 25 mL reaction tube, and use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, respectively Add 20 mg of different commercial lipases and react for 16 h at 600 rpm and 50 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: when Pseudomonas cepacia lipase is used as a catalyst, it preferentially recognizes ( R )-2-(3-chlorophenyl) heptyl propionate, its ee p 98.16%, c was 11.09%.
Embodiment 2
[0028] Put 0.020 mmol of different kinds of racemic 2-(3-chlorophenyl)propionate in a 25 mL reaction tube, and use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, Add 20 mg of Pseudomonas cepacia lipase and react for 15 h at 600 rpm and 40 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: when the substrate is 2-(3-chlorophenyl) heptyl propionate, its ee p 98.20%, c was 7.26%.
Embodiment 3
[0030] Put 0.020 mmol of racemic heptyl 2-(3-chlorophenyl)propionate in a 25 mL reaction tube, use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, add 20 mg of Pseudomonas cepacia lipase was reacted for 4 h at 600 rpm and 70 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: its ee p 98.82%, c was 10.31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com