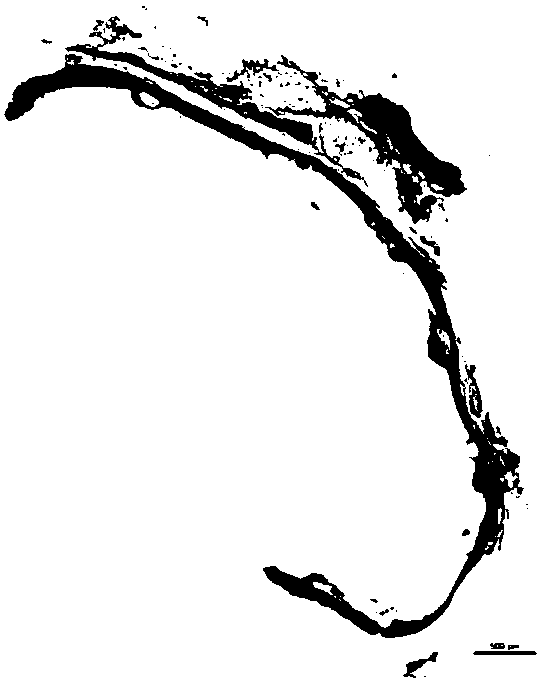
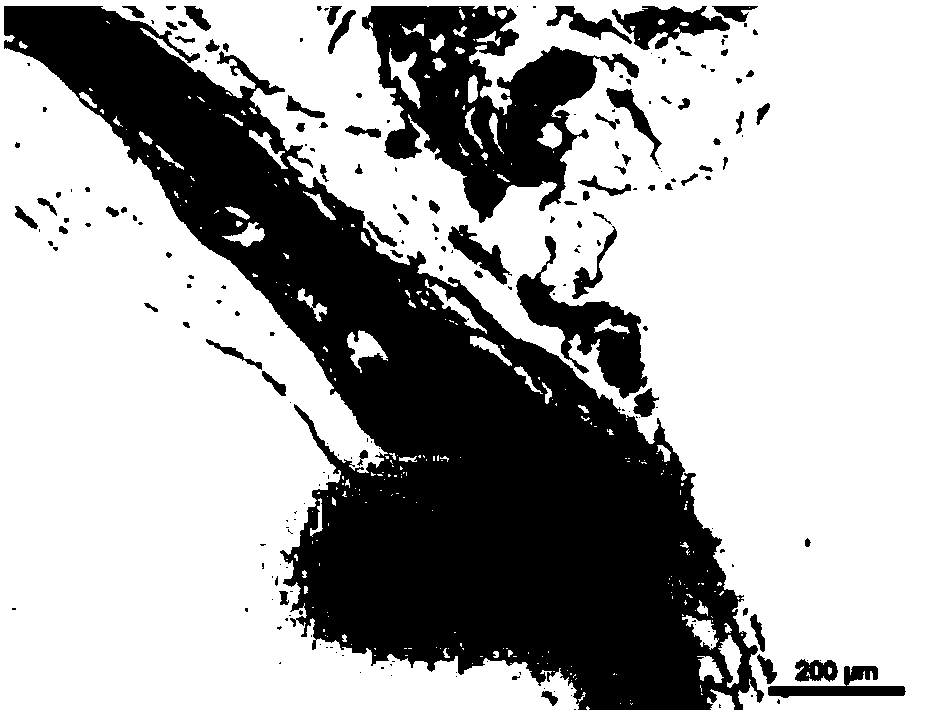
Absorbable iron-based instrument
An iron-based and device technology, applied in the field of absorbable iron-based devices, can solve the biological abnormalities of human tissue, affect the mechanical properties of iron-based devices, and cause biological safety risks, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]Polylactic acid was separated and purified by GPC to obtain polylactic acid with a residual monomer mass fraction of 0.1%. Polylactic acid with a residual monomer mass fraction of 0.1% was dissolved in ethyl acetate, and then sprayed on the surface of an iron-based alloy stent with a carbon content of 2.11 wt%, to obtain the degradable polyester-coated iron-based alloy of Example 1 Stent. In the degradable polyester-coated iron-based alloy vascular stent of this embodiment, the average thickness of the degradable polyester coating is 5 microns. The mass ratio of the degradable polyester to the iron-based matrix is 1:80.
[0056] Using the same material and method, make three identical stents, and randomly put one of the stents into a sample bottle, and add physiological saline to make it just completely submerge the stent. Place the sample bottle in a constant temperature water bath environment at 36.5°C to 37.5°C, and shake at a speed of 40-80 rpm. Then at day 3, t...
Embodiment 2
[0060] The polylactic acid is separated and purified by GPC method to obtain the preferred polylactic acid with a mass fraction of low molecular weight fractions of less than 100,000 and a mass fraction of residual monomers of 1.0%. The obtained preferred polylactic acid was dissolved in ethyl acetate, and sprayed on the surface of the pure iron stent to obtain the degradable polyester-coated pure iron stent of Example 2. In this embodiment, the average thickness of the degradable polyester coating is 5 microns. The mass ratio of the degradable polyester to the iron-based matrix is 1:100.
[0061] The degradable polyester-coated pure iron stent provided in Example 2 was implanted into the abdominal aorta of New Zealand rabbits. The stent and its surrounding vascular tissue were taken out 7 days after implantation. Cut and flatten the stent with vascular tissue in the axial direction, use a microscope to observe the appearance integrity of the stent, and observe whether the...
Embodiment 3
[0064] The polylactic acid is separated and purified by an ultrafiltration membrane filtration method to obtain the preferred polylactic acid with a residual monomer mass fraction of 0.05%. The obtained preferred polylactic acid was dissolved in ethyl acetate, and then sprayed on the surface of the iron-based alloy stent to obtain the degradable polyester-coated iron-based alloy stent of Example 3. In this embodiment, the average thickness of the degradable polyester coating is 2 microns. The mass ratio of the degradable polyester to the iron-based matrix is 1:200.
[0065] The degradable polyester-coated iron-based alloy stent provided in Example 3 was implanted into the abdominal aorta of New Zealand rabbits. The stent and its surrounding vascular tissue were taken out 7 days after implantation. Cut and flatten the stent with vascular tissue in the axial direction, use a microscope to observe the appearance integrity of the stent, and observe whether there is serious loc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com