Recombinant collagen hydrogel wound dressing and preparation method and application thereof
A technology for reconstituting collagen and wound dressings, applied in pharmaceutical formulations, pharmaceutical sciences, bandages, etc., can solve the problems of cytotoxicity, complicated preparation steps, etc., and achieve the effects of good biocompatibility, simple preparation, and reduction of scar formation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
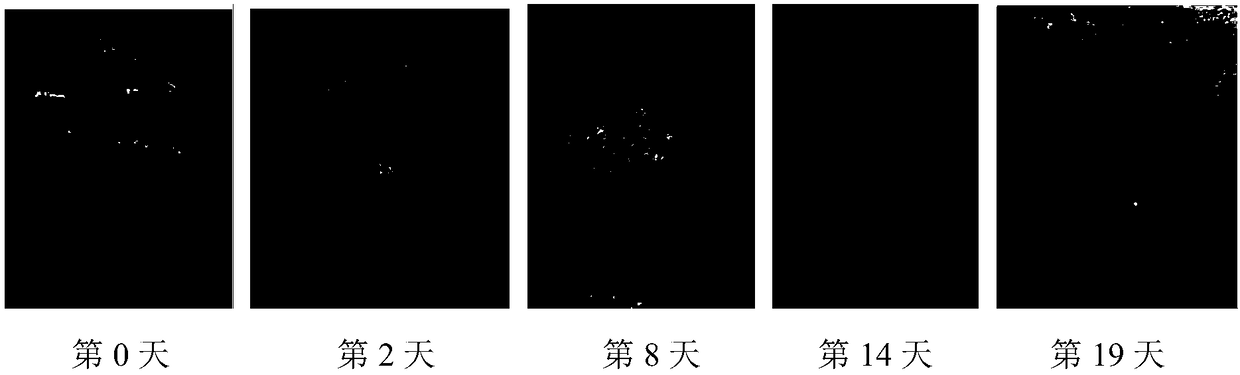
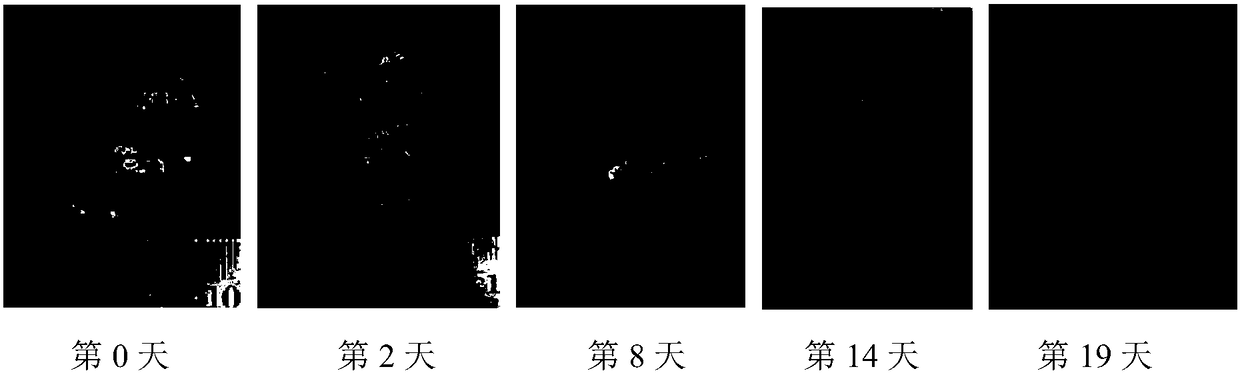
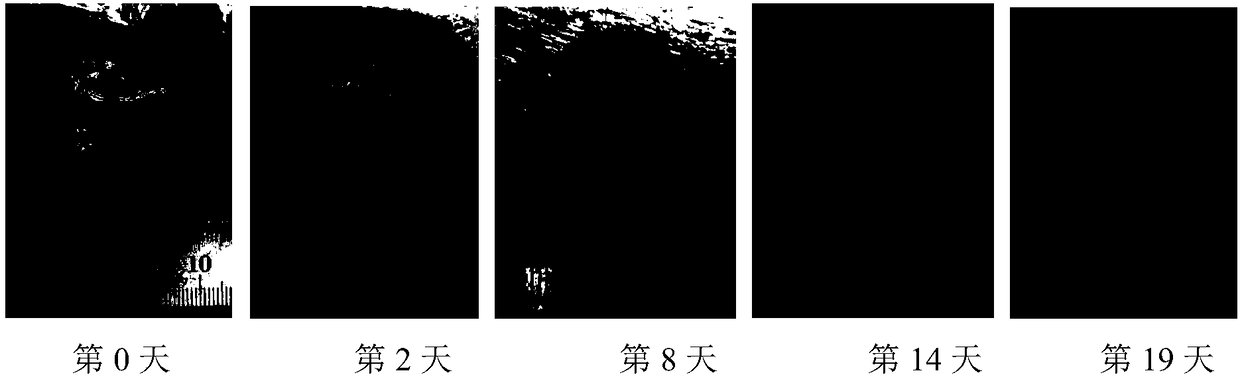
Image
Examples
Embodiment 1
[0029] Weigh 9.0 g of sodium chloride and dissolve it in 1000 mL of deionized water, stir until completely dissolved, and prepare a 0.9% sodium chloride solution. Weigh 5.0 g of sodium alginate, add 94 mL of the 0.9% sodium chloride solution prepared above to fully swell. After the sodium alginate is completely swollen, add 1.0 g of recombinant collagen and stir well until the recombinant collagen is completely dissolved and mixed evenly. The homogeneously mixed hydrogel is sterilized by high pressure steam to prepare a sterile recombined collagen hydrogel wound dressing.
Embodiment 2
[0031] Weigh 9.0 g of sodium chloride and dissolve it in 1000 mL of deionized water, stir until completely dissolved, and prepare a 0.9% sodium chloride solution. Weigh 4.0 g of sodium carboxymethylcellulose, add 95 mL of the 0.9% sodium chloride solution prepared above to fully swell. After the sodium carboxymethyl cellulose is completely swollen, add 1.0 g of recombinant collagen and stir well until the recombinant collagen is completely dissolved and mixed evenly. The homogeneously mixed hydrogel is sterilized by high pressure steam to prepare a sterile recombined collagen hydrogel wound dressing.
Embodiment 3
[0033] Weigh 9.0 g of sodium chloride and dissolve it in 1000 mL of deionized water, stir until completely dissolved, and prepare a 0.9% sodium chloride solution. Weigh 3.0 g of xanthan gum, add 96 mL of the 0.9% sodium chloride solution prepared above to fully swell. After the xanthan gum is completely swollen, add 1.0 g of recombinant collagen and stir well until the recombinant collagen is completely dissolved and mixed evenly. The homogeneously mixed hydrogel is sterilized by high pressure steam to prepare a sterile recombined collagen hydrogel wound dressing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com