Coumarin thiocarbazone derivatives as well as preparation method and application thereof
A technology of coumarin thiocarbazone and derivatives, which is applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problems of few probes, limited number of ratiometric fluorescent probes, complex synthesis process, etc., and achieve The effect of strong selectivity, wide potential application value, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Coumarin Thiocarbazone Derivatives
[0033]
[0034] Dissolve 2.59g of 3-acetyl-7-diethylaminocoumarin in 100mL of ethanol, then add 1.06g of thiocarbazide, reflux under normal pressure and stir for 2h, cool to room temperature, precipitate a large amount of solid, filter under reduced pressure, The filter residue was washed with ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 75%.
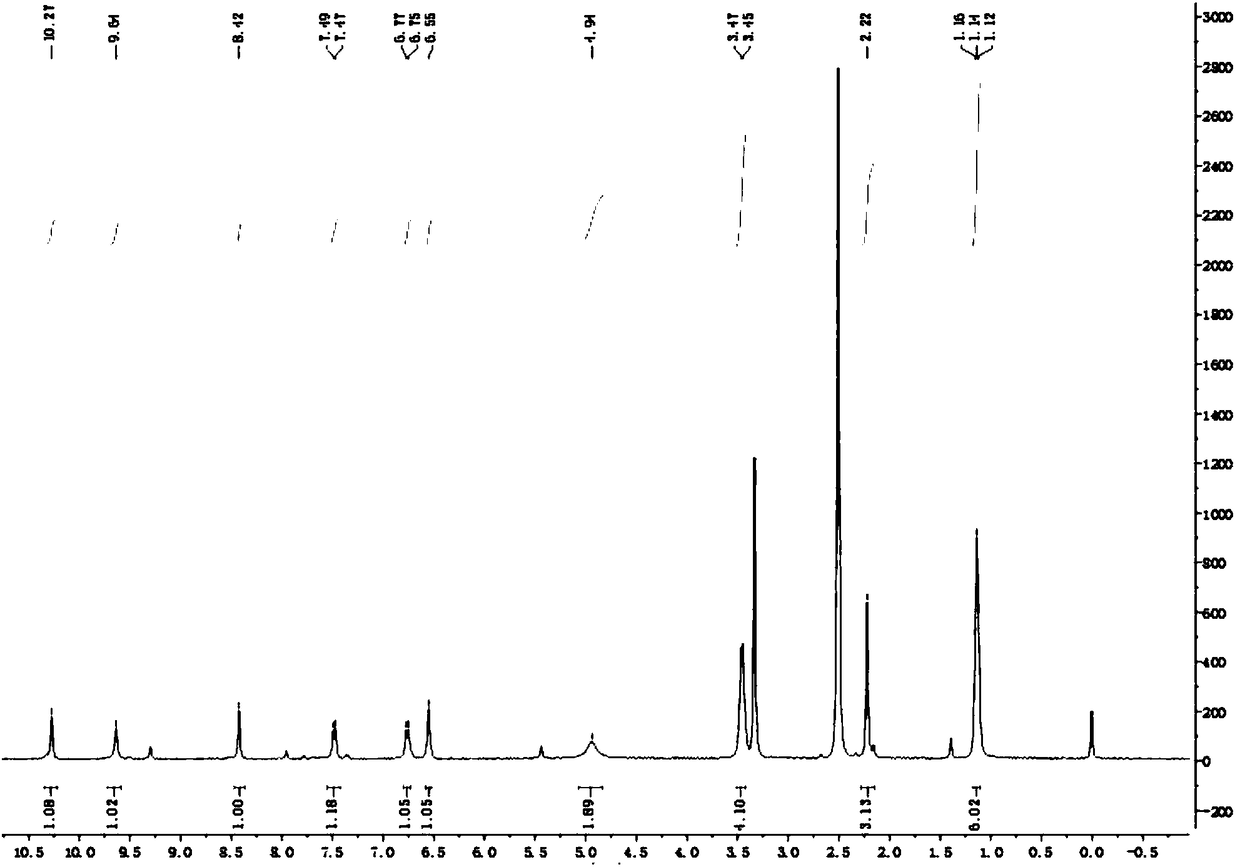
[0035] The prepared coumarin thiocarbazone derivatives were analyzed by nuclear magnetic resonance spectroscopy, and the results were as follows:
[0036] 1 H NMR (400MHz, d 6-DMSO)δ:10.27(1H,s,NH),9.64(1H,s,NH),8.42(1H) / 7.47-7.49(1H) / 6.75-6.77(1H) / 6.55(1H) for Ar-H ,4.94(2H,s,NH 2 ),3.43-3.49(4H,q,2CH 2 ),2.22(3H,s,CH 3 ),1.12-1.16(6H,t,2CH 3 ), see the specific NMR spectrum figure 1 ;
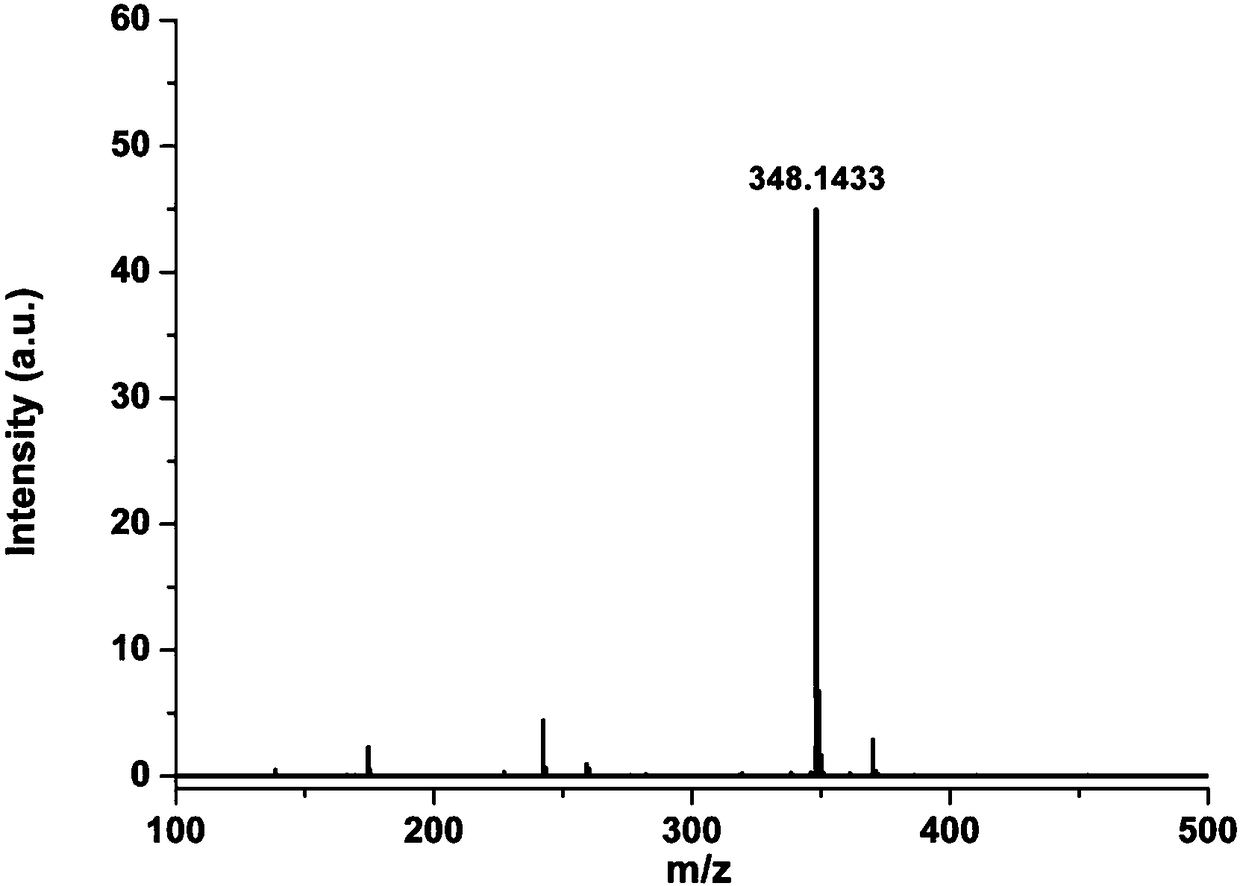
[0037] Mass Spectrum: ESI-MS: m / z=348.1433 for [M+H] + .Specific mass spectrogram see figure 2 .
Embodiment 2
[0039] Dissolve 5.18g of 3-acetyl-7-diethylaminocoumarin in 200mL of ethanol solution, then add 2.12g of thiocarbazide, reflux and stir for 4 hours under normal pressure, cool to room temperature, a large amount of solids are precipitated, and filter under reduced pressure , the filter residue was washed with ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 78%.
Embodiment 3
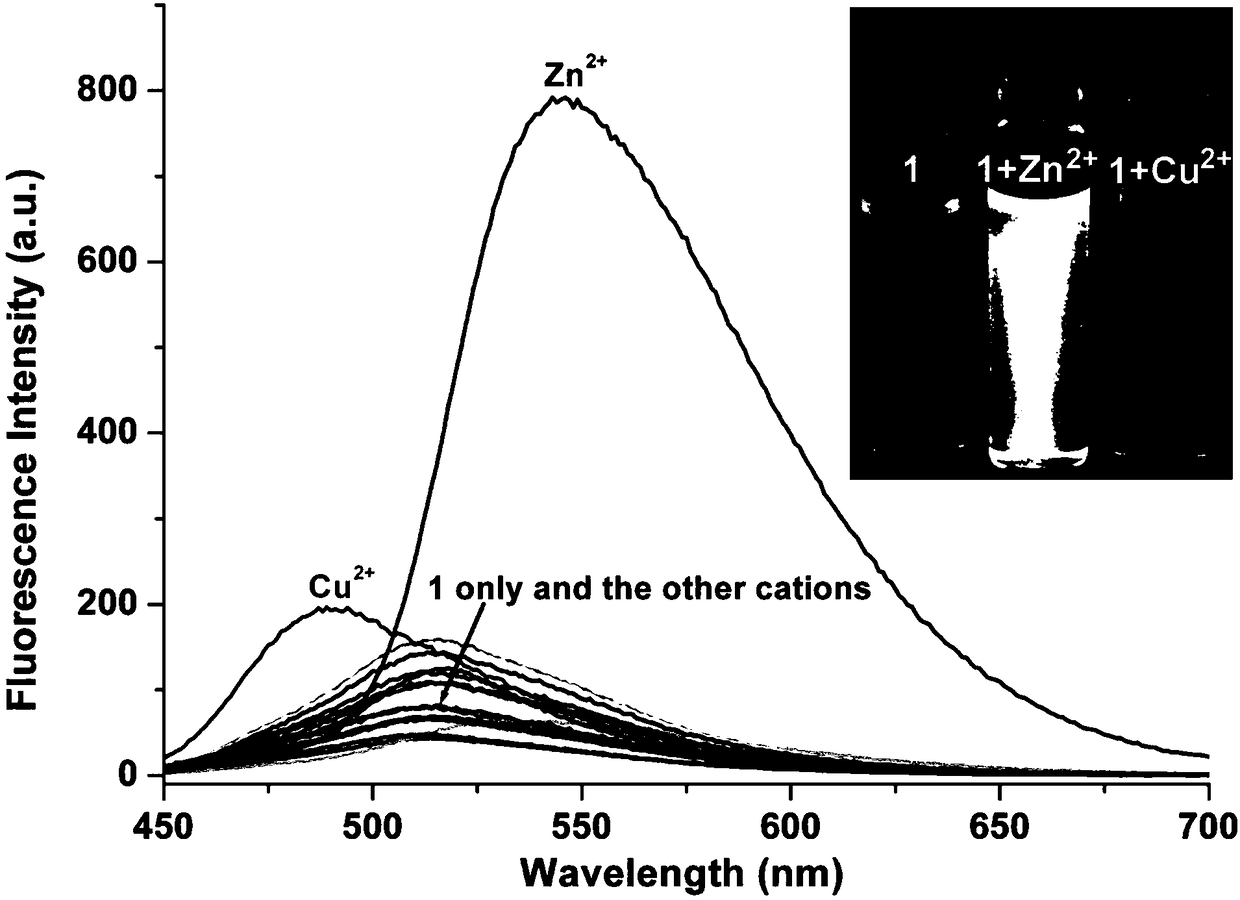
[0040] Example 3 Determination of Optical Properties of Coumarin Thiocarbazone Derivatives to Copper Ions and Zinc Ions
[0041] The coumarin thiocarbazone derivatives prepared in the above-mentioned embodiment 1 were used as fluorescent probes in DMF / H 2 In O(9 / 1,v / v) medium, the molar concentration is 5×10 -6 mol / L solution, respectively, at a molar concentration of 1×10 -5 mol / L Ag + , Al 3+ , Ca 2+ , Cd 2+ ,Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2+ , K + , Mg 2+ ,Mn 2+ , Na + , Ni 2+ ,Pb 2 ,Zn 2+ Add an equal amount of the above-mentioned fluorescent probe solution in the solution of metal ions, and adopt a fluorescence spectrometer to carry out fluorescence spectrum analysis (excitation wavelength is 420nm) to it respectively, and the fluorescence spectrum diagram of gained is shown in image 3 . pass image 3 It can be seen that the coumarin thiocarbazone derivative prepared in Example 1 of the present invention is used as a fluorescent probe to interact w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com