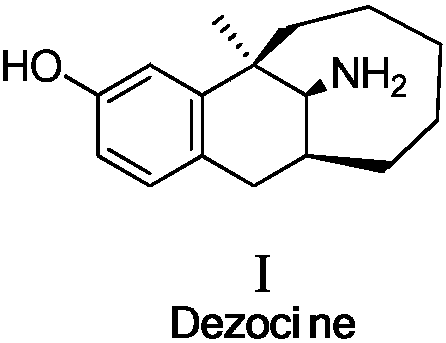
Asymmetric synthesis method of dezocine key intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of pharmaceutical compounds, can solve the problem that the racemization resolution process cannot be advanced, and achieve the effects of low cost, simple steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
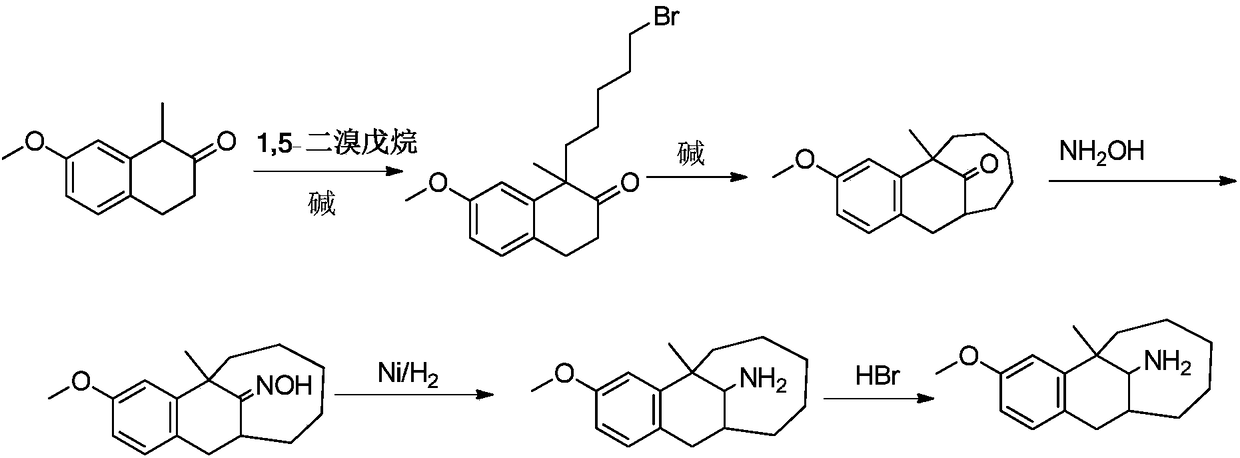
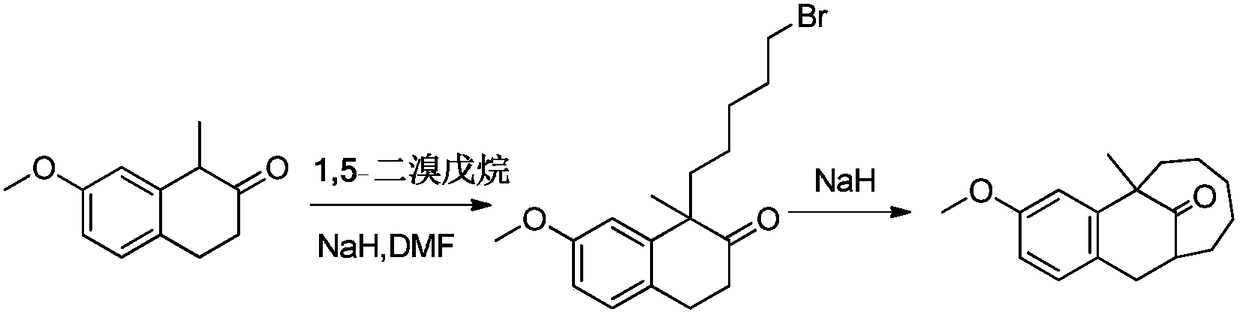
[0053] Preparation of (1R)-1-(5-bromopentyl)-7-methoxy-1-methyl-tetralone
[0054] In a 250ml three-necked flask, 7-methoxy-1-methyl-2-tetralone (2.5g, 13mmol), 1,5-dibromopentane (7.55g, 32.8mmol), cinchoni Butyl derivative catalyst (0.75g, 1.3mmol R is 2,4-difluorobenzyl, X is Br) and methyl tert-butyl ether (150ml, 55V), under nitrogen protection, under dark conditions, add 40wt% of KOH aqueous solution (KOH 7.3g, 130mmol), the reaction temperature is controlled at 5-15°C, after 3 hours of reaction, the liquids are separated, the aqueous phase is extracted three times with methyl tert-butyl ether (10ml each time), the organic phases are combined, and 3% The organic phase was washed with HCl; the aqueous phase was discarded, and the organic phase was washed with saturated NaCl solution until pH = 7.0-8.0; the organic phase was dried over anhydrous sodium sulfate. After concentrating under reduced pressure, 3.4 g of oil was obtained, with a yield of 78%; [α] D 20 =+24 (c=1...
Embodiment 2
[0058] Preparation of (1R)-1-(5-bromopentyl)-7-methoxy-1-methyl-tetralone
[0059] In a 500ml three-necked flask, 7-methoxy-1-methyl-2-tetralone (5.0g, 26.3mmol), 1,5-dibromopentane (15.1g, 65.7mmol), cinchor Nitin derivative catalyst (0.75g, 1.3mmol, R is 2-fluoro-4-trifluoromethylbenzyl, X is BF 4 ) and dichloromethane (150ml, 30V), under nitrogen protection, under dark conditions, 10wt% LiOH aqueous solution (LiOH 9.4g, 394.5mmol) was added dropwise, the reaction temperature was controlled at 5-15°C, after 6h of reaction, the liquid separation , the aqueous phase was extracted three times with dichloromethane (25ml each time), the organic phases were combined, and the organic phase was washed with 1% HCl; the aqueous phase was discarded, and the organic phase was washed to pH=7.0-8.0 with saturated NaCl solution; anhydrous sodium sulfate The organic phase is dried. After concentrating under reduced pressure, 6.7 g of oil was obtained, with a yield of 76%; [α] D 20 =+25(...
Embodiment 3
[0061] Preparation of (1R)-1-(5-bromopentyl)-7-methoxy-1-methyl-tetralone
[0062] In a 5L three-necked flask, 7-methoxy-1-methyl-2-tetralone (50g, 263mmol), 1,5-dibromopentane (151g, 657.5mmol), cinchonidine derivatized Catalyst (14.1g, 26.3mmol, R is 3-fluoro-4-trifluoromethylbenzyl, X is Br) and toluene (1.65L, 35V), nitrogen protection, under dark conditions, drop 30wt% of CsOH aqueous solution (CsOH 587g, 3.945mol), the reaction temperature is controlled at 10-15°C, after 1h of reaction, the liquid is separated, the aqueous phase is extracted three times with toluene (250ml each time), the organic phase is combined, and the organic phase is washed with 5% HCl 2 times; the aqueous phase was discarded, and the organic phase was washed with saturated NaCl solution to pH=7.0-8.0; the organic phase was dried over anhydrous sodium sulfate. After concentrating under reduced pressure, 72.2 g of oil was obtained, with a yield of 81%; [α] D 20 =+23 (c=1.0.CHC1 3 ), the HPLC det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com