Method of utilizing micro channel reactor to prepare fluorine containing chalcone derivatives
A technology of microchannel reactor and fluorine-containing chalcone, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as low product quality, shorten reaction time, and increase safety. Achieve the effect of improving reaction efficiency, shortening reaction time and increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
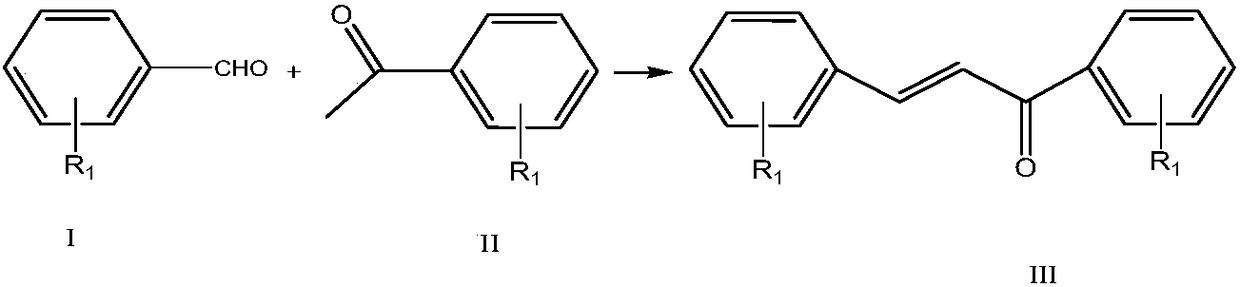
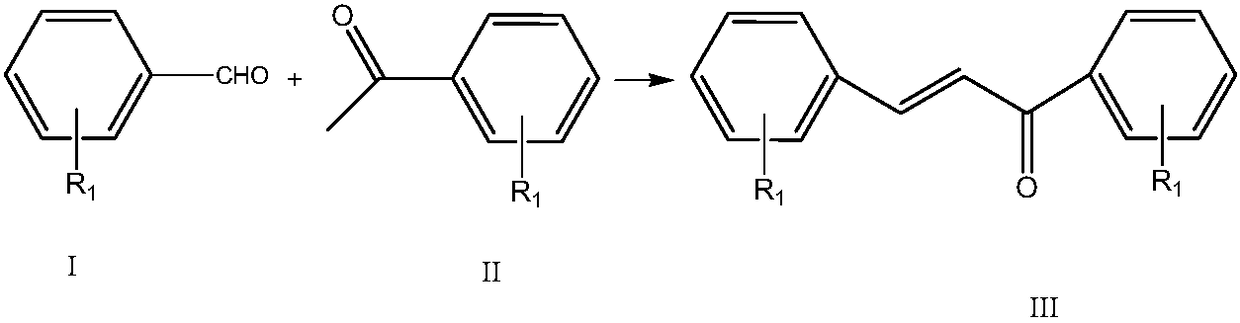
[0031] Example 1 Synthesis of 4'-fluorochalcone by reaction of 4-fluorobenzaldehyde and acetophenone
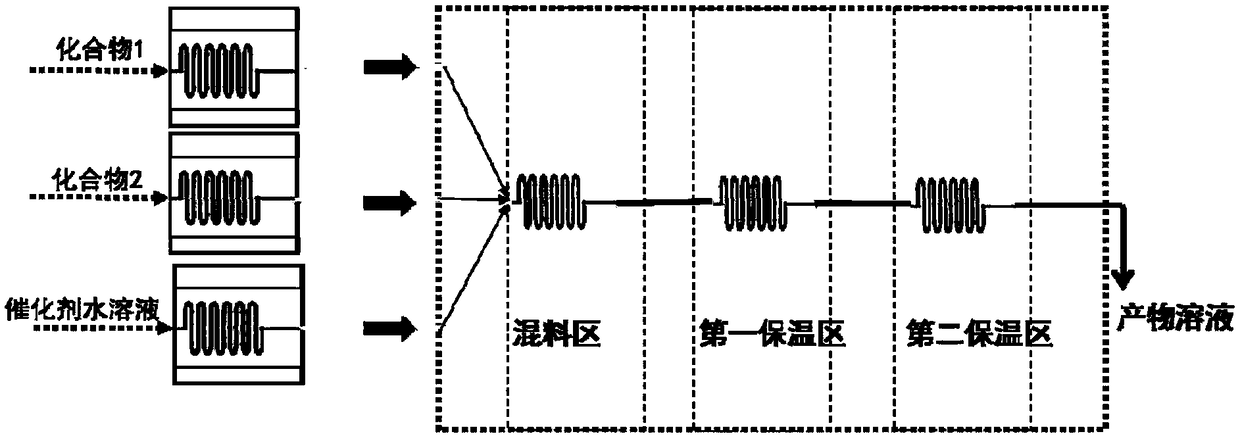
[0032] 4-fluorobenzaldehyde is formulated with ethanol into a 0.5mol / L solution, acetophenone is formulated with ethanol into a 0.5mol / L solution, sodium hydroxide is formulated into a 0.2mol / L aqueous solution, 4-fluorobenzaldehyde / ethanol solution, benzene Ethanone / ethanol solution and sodium hydroxide aqueous solution were pumped into the microchannel reactor at flow rates of 15ml / min, 18ml / min, and 3ml / min respectively, the temperature in the first temperature zone was set to 35°C, the residence time was 30s, and the second temperature zone was set at 35°C. The zone temperature is 60°C and the residence time is 60s. The product flows out from the outlet, and after acidification, it is refined to obtain 4'-fluorochalcone. The selectivity of 4-fluorobenzaldehyde is 98.7%, the conversion rate is 98.1%, and the yield is 96.8%.
Embodiment 2
[0033] Example 2 Synthesis of 4-trifluoromethyl-4'-fluorochalcone by reaction of 4-fluorobenzaldehyde and 4-trifluoromethylacetophenone
[0034] 4-fluorobenzaldehyde is formulated with isopropanol into a 0.6mol / L solution, 4-trifluoromethylacetophenone is formulated with a 0.5mol / L solution with isopropanol, and sodium hydroxide is formulated with a 0.1mol / L aqueous solution, 4-fluorobenzaldehyde / ethanol solution, 4-trifluoromethylacetophenone / ethanol solution, sodium hydroxide aqueous solution are pumped into the microchannel reactor with the flow velocity of 10ml / min, 10ml / min, 2ml / min respectively, the first The temperature in the temperature zone is set at 40°C, and the residence time is 40s. The temperature in the second temperature zone is 60°C, and the residence time is 70s. The product flows out from the outlet, and after acidification, it is refined to obtain 4-trifluoromethyl-4'-fluorochalcone , The selectivity of 4-fluorobenzaldehyde was 98.2%, the conversion rate w...
Embodiment 3
[0035] Embodiment 3 benzaldehyde reacts with 3-fluoroacetophenone to synthesize 3-fluorochalcone
[0036]Benzaldehyde is formulated with methanol into a 1.0mol / L solution, 3-fluoroacetophenone is formulated with methanol into a 0.5mol / L solution, sodium hydroxide is formulated into a 0.4mol / L aqueous solution, benzaldehyde / methanol solution, 3-fluorobenzene Acetone / ethanol solution and sodium hydroxide aqueous solution were pumped into the microchannel reactor at flow rates of 20ml / min, 48ml / min, and 5ml / min respectively, the temperature in the first temperature zone was set to 25°C, and the residence time was 30s. The zone temperature is 45°C, the residence time is 90s, and the product flows out from the outlet, and is acidified and refined to obtain 3-fluorochalcone. The selectivity of benzaldehyde is 98.9%, the conversion rate is 98.2%, and the yield is 97.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com