Method and system for producing D-phenylalanine
A technology of phenylalanine and production process, applied in the field of producing D-phenylalanine, can solve the problems of high price, unenvironmental protection and high cost, and achieve the effects of low cost, time saving, simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 A kind of processing method of producing D-phenylalanine, comprises the following steps:
[0020] 1) Add 1300kg of acetic acid and 5kg of salicylaldehyde into the dry first reaction kettle, stir evenly, then add 500kg of raw material L-phenylalanine, raise the temperature to 70°C, and keep stirring for 1 hour. Concentrated and centrifuged to obtain DL-phenylalanine 450kg;
[0021] 2) Add the obtained 450kgDL-phenylalanine into the second reaction kettle, then add 1500kg of propionic acid and 410kg of D-tartaric acid, heat up to 65°C, keep stirring for 4 hours, after the reaction is completed, concentrate and centrifuge to obtain D- Phenylalanine·D-tartrate double salt 810kg.
[0022] 3) Add 810kg of the obtained D-phenylalanine·D-tartrate double salt into the third reaction kettle, add 1620kg of water to dissolve it, and then add ammonia water to adjust its pH to the isoelectric point of D-phenylalanine (5.5);
[0023] 4) Send the D-phenylalanine·D-tartra...
Embodiment 2
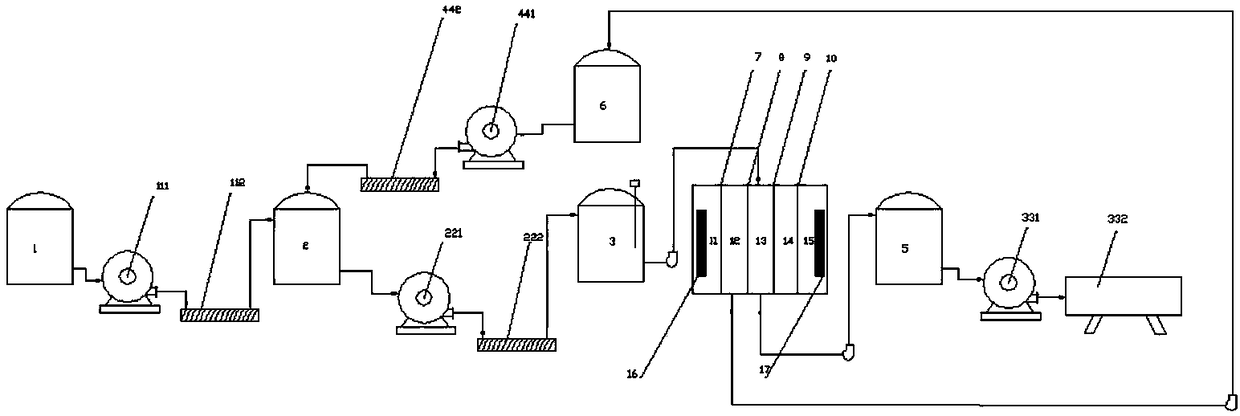
[0025] see figure 1 , a process system for producing D-phenylalanine, comprising the first reactor 1, the first centrifuge 111, the first conveyor 112, the second reactor 2, the second centrifuge 221, the second Conveyor 222 and the third reaction kettle 3, the third reaction kettle 3 is connected to the inlet of the raw water chamber 13 of the electrodialysis device through a feed pipe, and the outlet of the raw water chamber 13 of the electrodialysis device is connected to the first concentrated The crystallization tank 5 is connected, and the first concentrated crystallization tank 5 is connected with the third centrifuge 331 and the vacuum dryer 332 successively, and the outlet of the anion concentration chamber 12 of the electrodialysis device is connected with the second concentrated crystallization tank 6 through the concentrated water discharge pipe. The second concentrated crystallization tank 6 is connected with the fourth centrifuge 441 , and the fourth centrifuge 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com