Kit for evaluating curative effect after intensive treatment of pulmonary tuberculosis and prognosis and applications thereof
A technology for intensive treatment and tuberculosis, applied in disease diagnosis, biological testing, material inspection products, etc., can solve the problems of treatment evaluation, poor specificity, and long time consumption, and achieve good detection efficiency, high sensitivity and specificity, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Composition of curative effect evaluation and prognosis evaluation kit after intensive treatment of pulmonary tuberculosis
[0054] In this embodiment, the curative effect evaluation and prognosis evaluation kit after intensive treatment of pulmonary tuberculosis includes a microtiter plate, protein standard composition and biotin-labeled antibody composition, sample and antibody diluent, detergent, horseradish Peroxidase-labeled avidin, chromogenic substrate solution, stop solution and microtiter plate attachment, specifically:
[0055] (1) The enzyme plate includes: an enzyme plate coated with an anti-ANGT antibody, purchased from ray biotech company; an enzyme plate coated with an anti-CO7 antibody, an enzyme plate coated with an anti-PLMN antibody, purchased from From abcam company; coated with anti-APOC2 antibody microtiter plate, purchased from abnova company.
[0056] (2) The protein standard composition includes: ANGT, CO7, PLMN and APOC2 protein ...
Embodiment 2
[0069] Example 2: Establishment of the Logistic stepwise regression model of the efficacy evaluation and prognosis evaluation kit after intensive treatment of pulmonary tuberculosis
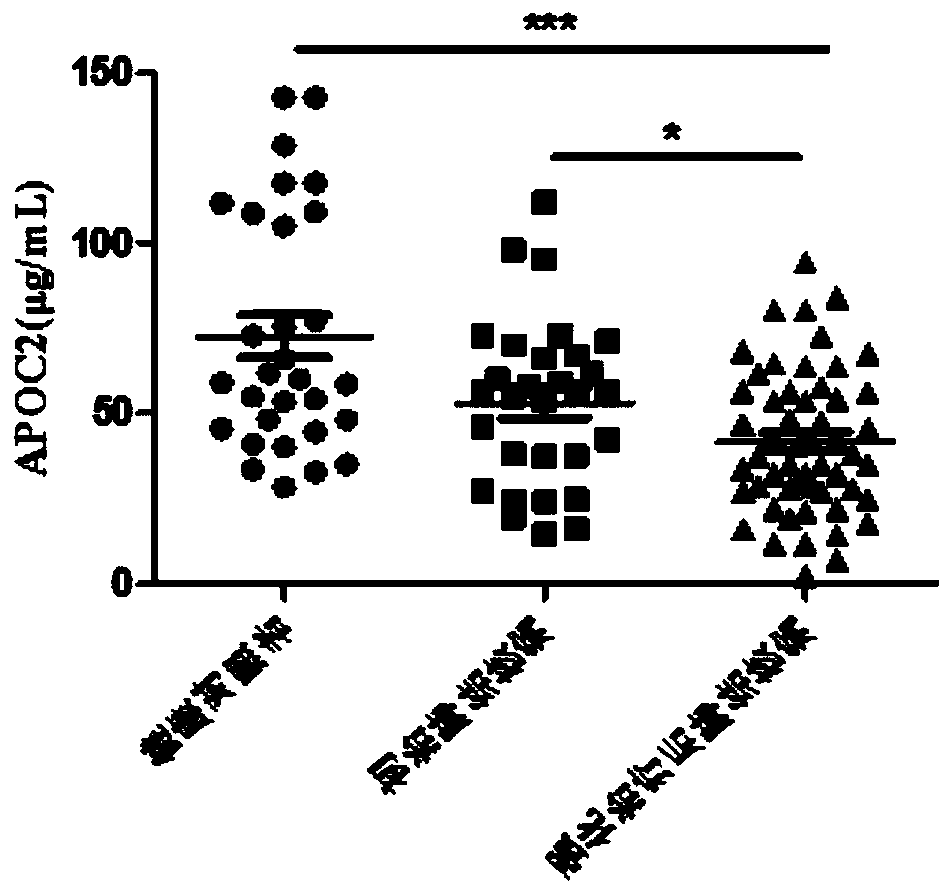
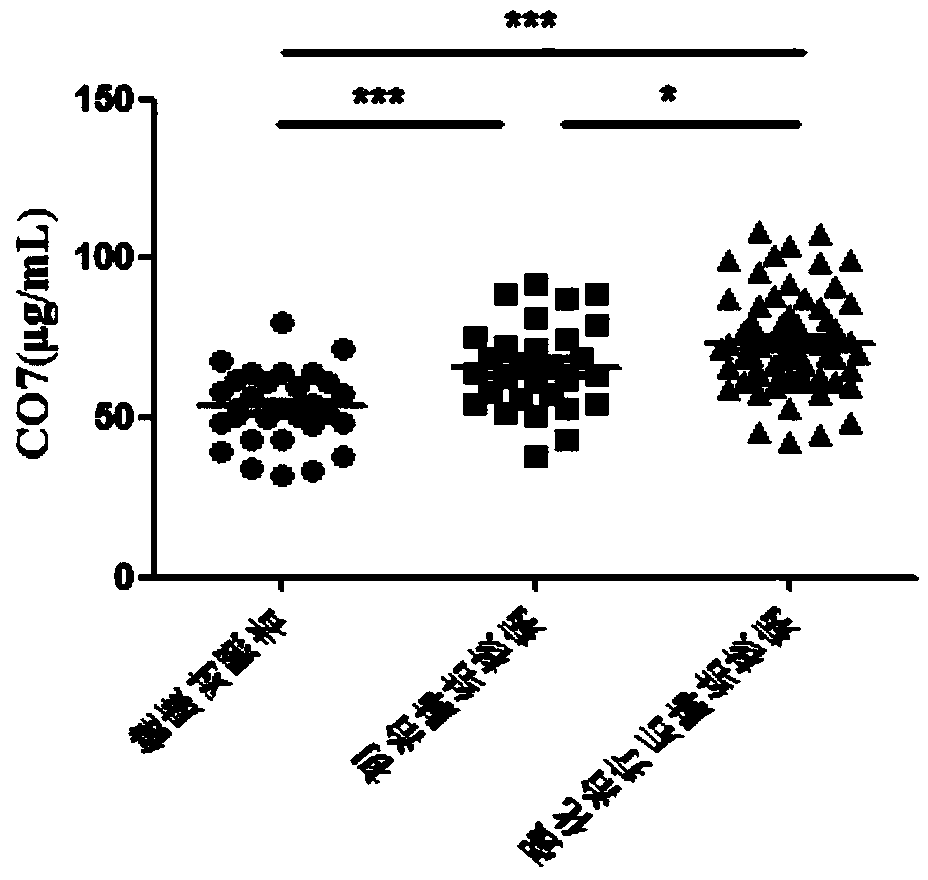
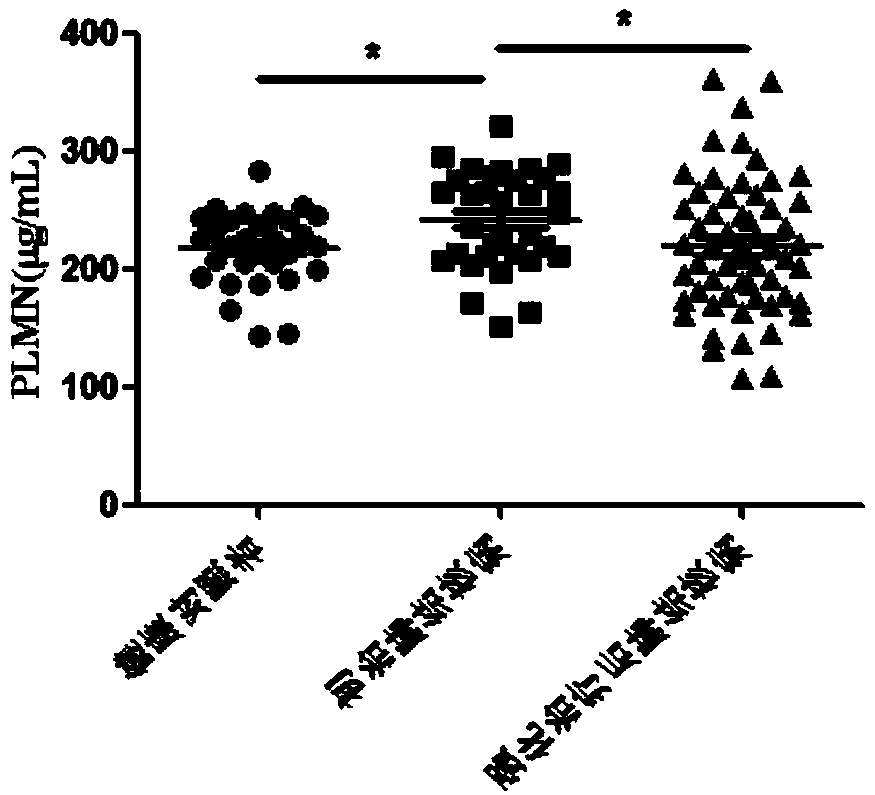
[0070] The curative effect evaluation and prognosis evaluation kits after intensive treatment of tuberculosis were used to detect the protein levels of ANGT, CO7, PLMN and APOC2 in the serum of healthy controls, newly treated tuberculosis patients, and tuberculosis patients after intensive treatment.
[0071] (1) Serum sample collection:
[0072] According to the diagnostic criteria of tuberculosis issued by the Ministry of Health of China, 48 cases of tuberculosis patients who were not drug-treated at the beginning of treatment, 81 cases of tuberculosis patients after intensive treatment, and 40 cases of healthy controls were collected. The subjects were excluded from tumors, extrapulmonary tuberculosis, diabetes, hepatitis B, AIDS, non-tuberculous mycobacteria, drug-resistant tuberculosis and o...
Embodiment 3
[0094] Example 3: Verification and application of the detection effect of the curative effect evaluation and prognosis evaluation kit after intensive treatment of pulmonary tuberculosis
[0095] Holdout verification: Serum samples were taken from healthy controls, newly treated pulmonary tuberculosis patients, and pulmonary tuberculosis patients after intensive treatment. The sample collection and dilution methods were the same as those described in Example 2.
[0096] Add the diluted sample to be tested into four kinds of enzyme-labeled plates coated with anti-ANGT antibody, CO7 antibody, PLMN antibody, and APOC2 antibody, affix the enzyme-labeled plate, and incubate at room temperature for 1-2.5 hours.
[0097] Discard the waste liquid, pat dry the microtiter plate, and wash 4 to 5 times with detergent. And add the corresponding freshly prepared diluted biotin-labeled antibody to each reaction well, affix the microplate plate, and incubate at room temperature for 1 hour.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com