Method of detecting protein charge variant and method for determining production process of bioproduct
A technology for biological products and variants, applied in the field of biomedicine, can solve the problems of uneven molecular size, charge, glycosylation modification, etc., and achieve the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The inventors explored a lot of test methods in the early stage (including mobile phase composition, mobile phase pH value, elution time and chromatographic column screening), and finally determined the key parameters of the test method, and successfully applied to the detection and analysis of protein charge variants. The determination of critical process parameters (CPPs) in the process sector provides a theoretical basis and reference to ensure the safety and effectiveness of clinical medication.
[0057] 1.1 Exploration of mobile phase components
[0058] Condition 1:
[0059] The composition of PIT mobile phase is shown in Table 4.
[0060] Table 4:
[0061]
[0062] The chromatographic conditions are shown in Table 5.
[0063] table 5:
[0064] conditional content
[0065] The elution program is shown in Table 6, and the elution time is 20 min.
[0066] Table 6:
[0067] time
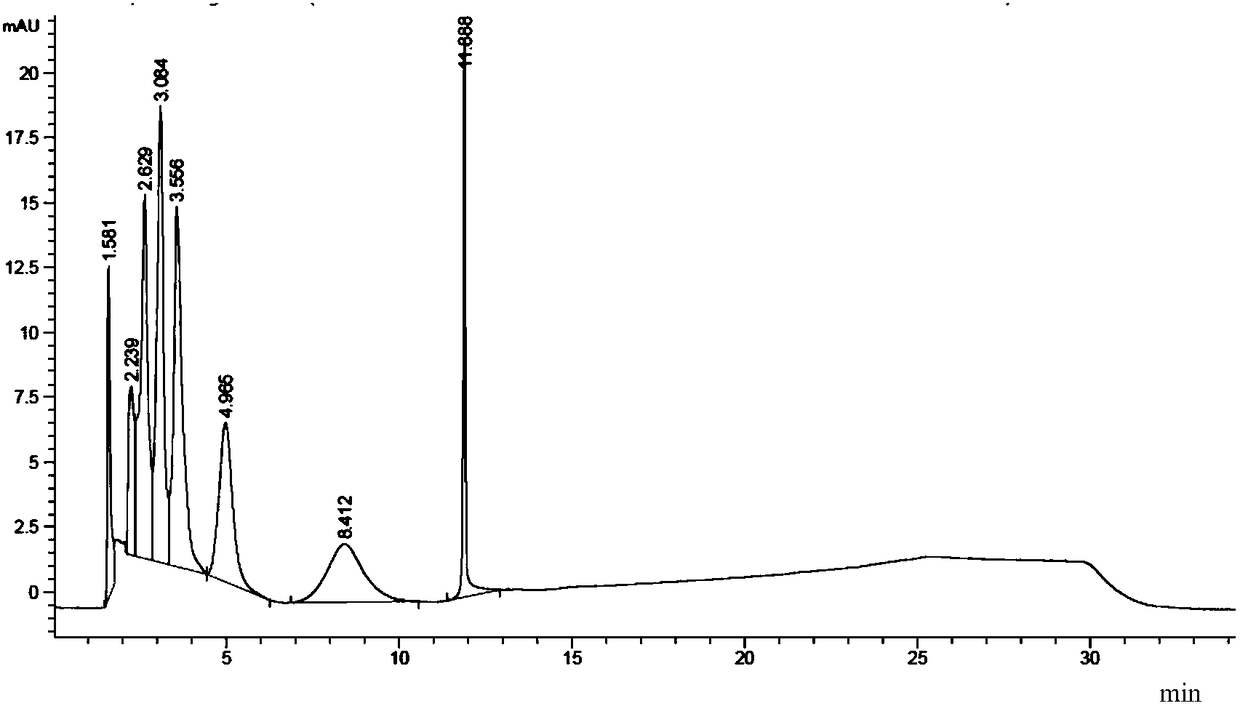
[0068] The obtained chromatogram is as figure 1 shown. T...
Embodiment 2
[0189] Embodiment 2 charge variant peak identification
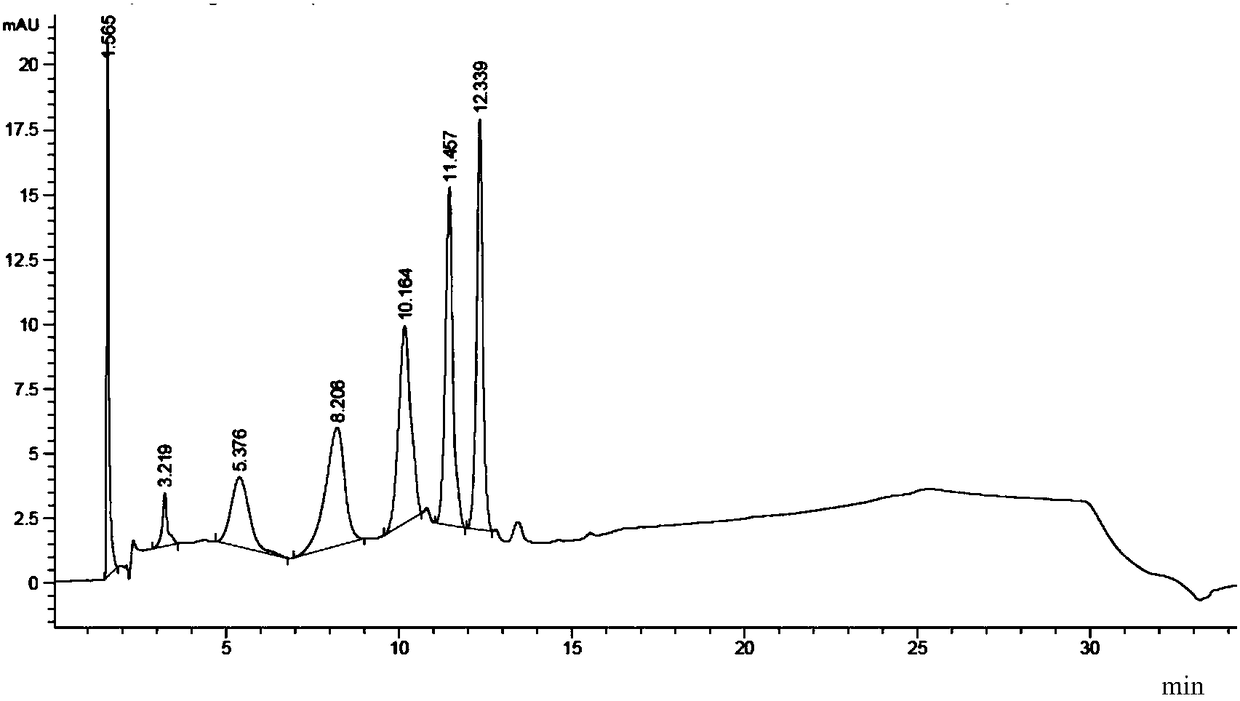
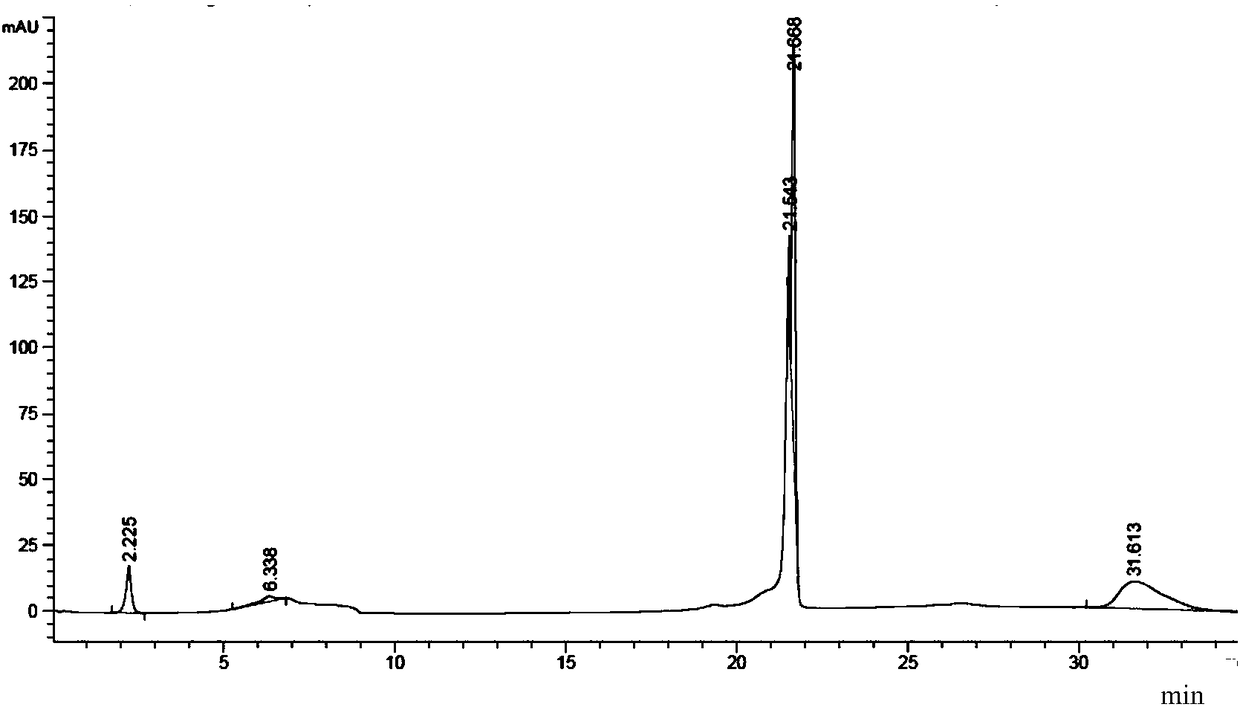
[0190] The inventor, on the basis of completely separating the variant peaks of the target protein (protein A, monoclonal antibody A), used sialidase (neuraminidase) digestion to identify the variant peaks. The identification results are as follows: Figure 12 and Figure 13 shown. in, Figure 12 The pH-HPLC chromatogram before protein A digestion is shown, Figure 13 The pH-HPLC chromatogram after protein A digestion is shown.
[0191] Depend on Figure 12 and Figure 13 The results showed that after the target protein A was digested with sialidase, all variant peaks disappeared before 21.2 minutes, indicating that the previous variant peaks were all acidic variant peaks, and it was speculated that the post-translational modification of the target protein A was mainly sialic acid modification .
[0192] According to the above test results, it is determined that the control range of the acidic charge variant of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com