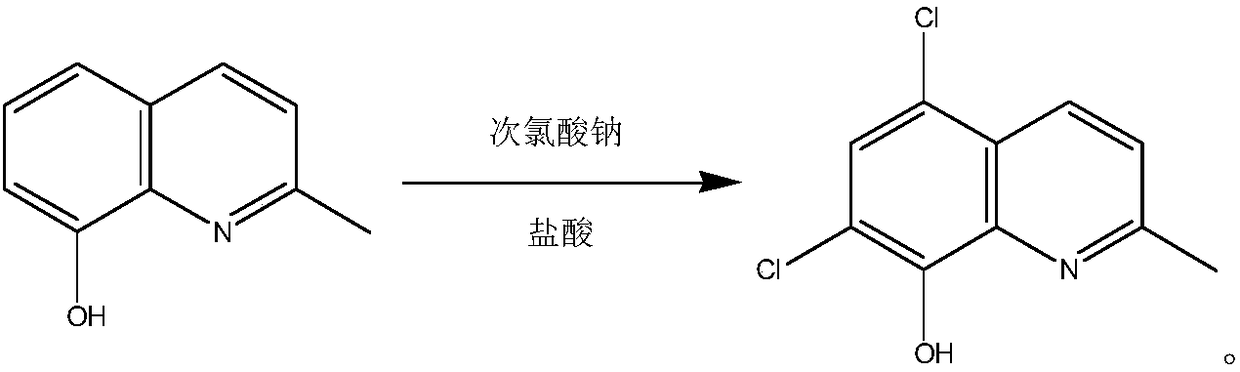
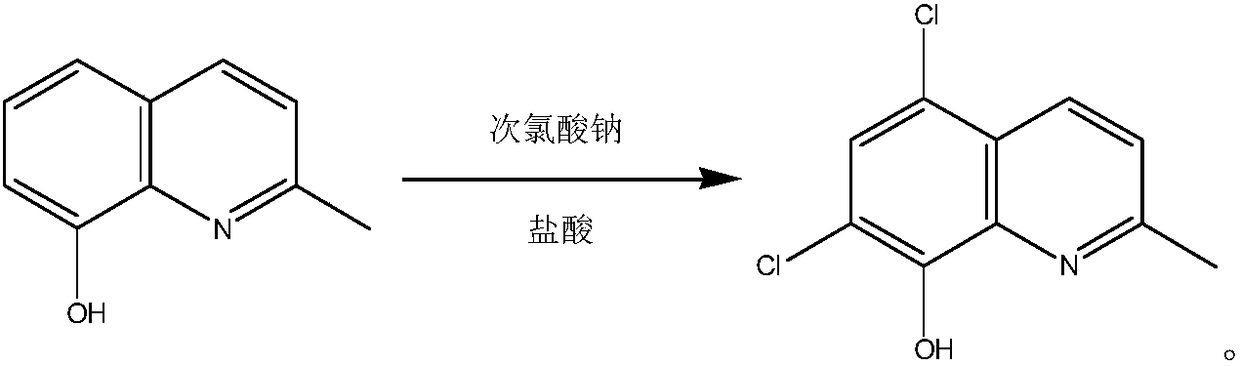
Process for synthesizing chlorquinaldol
A chloroquinaldol and process technology, applied in the field of drug synthesis, can solve problems such as environmental pollution, increase equipment investment, side reactions, etc., and achieve the effects of reducing production cost, less equipment investment, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 1000 ml three-necked flask, put 400 grams of 20wt.% hydrochloric acid and 20 grams of 8-hydroxy-2-methylquinoline, cool down to 5°C, add 187 grams of 10wt.% sodium hypochlorite solution dropwise, and control the dropping time 3 hours, during the dropwise addition, control the reaction temperature at 0-10°C, after the dropwise addition, keep it warm for 0.5 hours, and filter to obtain the wet product;
[0020] Put the wet product into 200 ml of purified water, adjust the pH value to 4-5 with sodium carbonate, filter with suction, rinse, and dry to obtain the crude product, which is refined with 200 ml of methanol and 200 ml of purified water to obtain 27.6 g of chloroquinaldol. Yield 96.5%, HPLC purity 99.93%.
Embodiment 2
[0022] In a 1000 ml there-necked flask, put 600 grams of 15wt.% hydrochloric acid and 20 grams of 8-hydroxy-2-methylquinoline, cool down to 15°C, add 187 grams of 10wt.% sodium hypochlorite solution dropwise, and control the dropping time 4 hours, during the dropwise addition, control the reaction temperature at 10-20°C, after the dropwise addition, keep it warm for 0.5 hours, and filter to obtain the wet product;
[0023] Put the wet product into 200 ml of purified water, adjust the pH value to 4-5 with sodium carbonate, suction filter, rinse, and dry to obtain the crude product, which is refined with 200 ml of methanol and 100 ml of purified water to obtain 26.6 g of chloroquinaldol. Yield 92.7%, HPLC purity 99.95%.
Embodiment 3
[0025] In a 1000 ml three-necked flask, put 300 grams of 30wt.% hydrochloric acid and 20 grams of 8-hydroxyl-2-methylquinoline, cool down to 5°C, add 196 grams of 10wt.% sodium hypochlorite solution dropwise, and control the dropping time 5 hours, during the dropwise addition, the reaction temperature was controlled at 0-10°C. After the dropwise addition was completed, keep the temperature for 0.5 hours, and filter to obtain the wet product;
[0026] Put the wet product into 200 ml of purified water, adjust the pH value to 4-5 with sodium carbonate, suction filter, rinse, and dry to obtain the crude product, which is refined with 200 ml of methanol and 300 ml of purified water to obtain 27.2 g of chloroquinaldol. Yield 95.3%, HPLC purity 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com