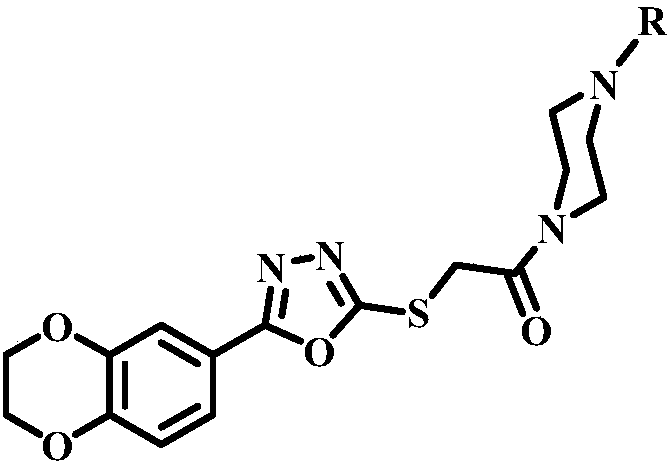
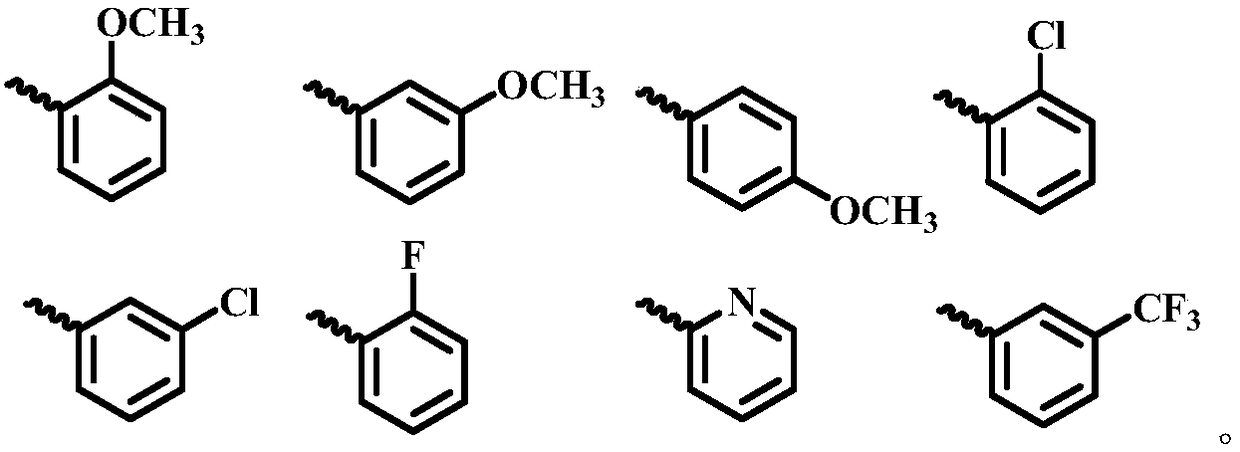
Piperazine amide compounds containing benzdioxan skeleton and preparation method and application thereof
A technology of benzodioxane and piperazine amide, which is applied in the field of piperazine amide compounds and their preparation, can solve the problems of no piperazine amide compounds, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 2-(5-(2,3-dihydrobenzo[1,4]dioxane)-1,3,4-oxadiazole-2-thione)-1-(4-(2-methoxy The preparation of base phenyl) piperazine) ethanone, its molecular formula is:
[0033]
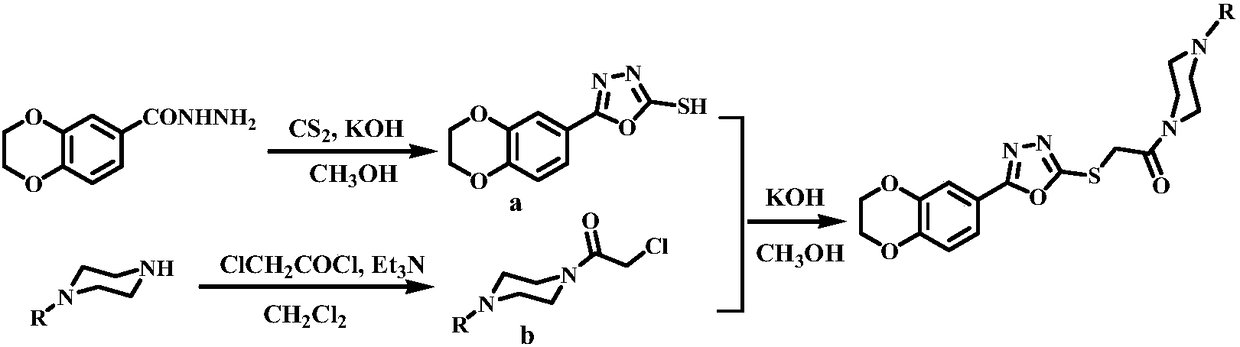
[0034] (1) Dissolve 10mmol of 1,4-benzodioxanecarbohydrazide and 12mmol of potassium hydroxide in 50mL of methanol, add 100mmol of carbon disulfide dropwise in an ice-water bath, and control the dropping within 15 minutes. After the dropwise addition of carbon disulfide, reflux React for 8 hours, spin to dry the solvent, add water to dissolve, filter, add hydrochloric acid to the filtrate, and filter to obtain product a;
[0035] (2) Dissolve 8mmol o-methoxyphenylpiperazine and 9.6mmol triethylamine in 32mL dichloromethane, slowly add 9.6mmol chloroacetyl chloride dropwise under ice-bath conditions, and control the dripping within 3 minutes; ice-bath conditions Under stirring reaction for 10 hours, filter to obtain product b;
[0036] (3) Dissolve 4mmol of product a, 4mmol of product b, and 6mmol o...
Embodiment 2
[0042] 2-(5-(2,3-dihydrobenzo[1,4]dioxane)-1,3,4-oxadiazole-2-thione)-1-(4-(3-methoxy The preparation of base phenyl) piperazine) ethanone, its molecular formula is:
[0043]
[0044] (1) Dissolve 10mmol of 1,4-benzodioxanecarbohydrazide and 13mmol of potassium hydroxide in 60mL of methanol, add 100mmol of carbon disulfide dropwise in an ice-water bath, and finish dropping within 18 minutes. After adding carbon disulfide dropwise, react under reflux for 9 hours, spin dry the solvent, add water to dissolve, filter, add hydrochloric acid to the filtrate to acidify, and filter to obtain product a;
[0045](2) 8 mmol of m-methoxyphenylpiperazine and 11 mmol of triethylamine were dissolved in 32 mL of dichloromethane, and 11 mmol of chloroacetyl chloride was slowly added dropwise under ice-bath conditions, and the drop was completed within 4 minutes. The reaction was stirred for 11 hours under ice-bath conditions, and filtered to obtain product b;
[0046] (3) 4mmol product a,...
Embodiment 3
[0052] 2-(5-(2,3-dihydrobenzo[1,4]dioxane)-1,3,4-oxadiazole-2-thione)-1-(4-(4-methoxy The preparation of base phenyl) piperazine) ethanone, its molecular formula is:
[0053]
[0054] (1) Dissolve 10mmol of 1,4-benzodioxanecarbohydrazide and 15mmol of potassium hydroxide in 70mL of methanol, add 100mmol of carbon disulfide dropwise in an ice-water bath, and finish dropping within 20 minutes. After adding carbon disulfide dropwise, reflux for 10 hours, spin to dry the solvent, add water to dissolve, filter, add hydrochloric acid to the filtrate to acidify, and filter to obtain product a;
[0055] (2) Dissolve 8 mmol of p-methoxyphenylpiperazine and 12 mmol of triethylamine in 32 mL of dichloromethane, slowly add 12 mmol of chloroacetyl chloride dropwise under ice-bath conditions, and finish the dropping within 5 minutes. Stir the reaction under ice bath conditions for 12 hours, filter to obtain product b;
[0056] (3) 4mmol product a, 4mmol product b, 6mmol potassium hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com