Anti-apoptotic protein PTD-FNK protein and method for preparing same
A technology of PTD-FNK and anti-apoptotic protein, which is applied in the field of anti-apoptotic protein PTD-FNK protein and its preparation, can solve the problems of hindering the application process of protein, restricting the mass production of protein, and large protein loss, etc., and achieve the meticulous preparation method Rigorous, highly repeatable, and expressive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
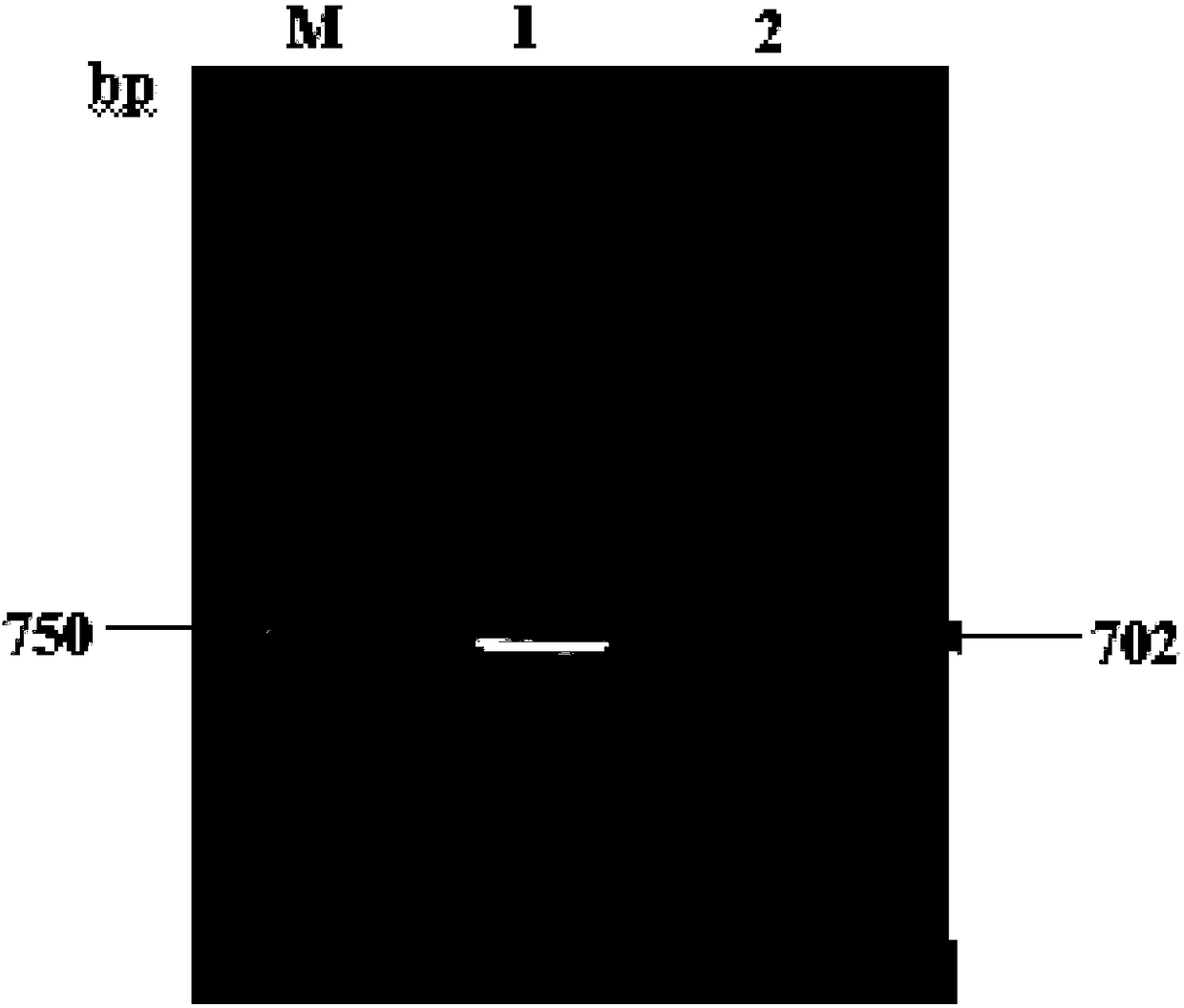
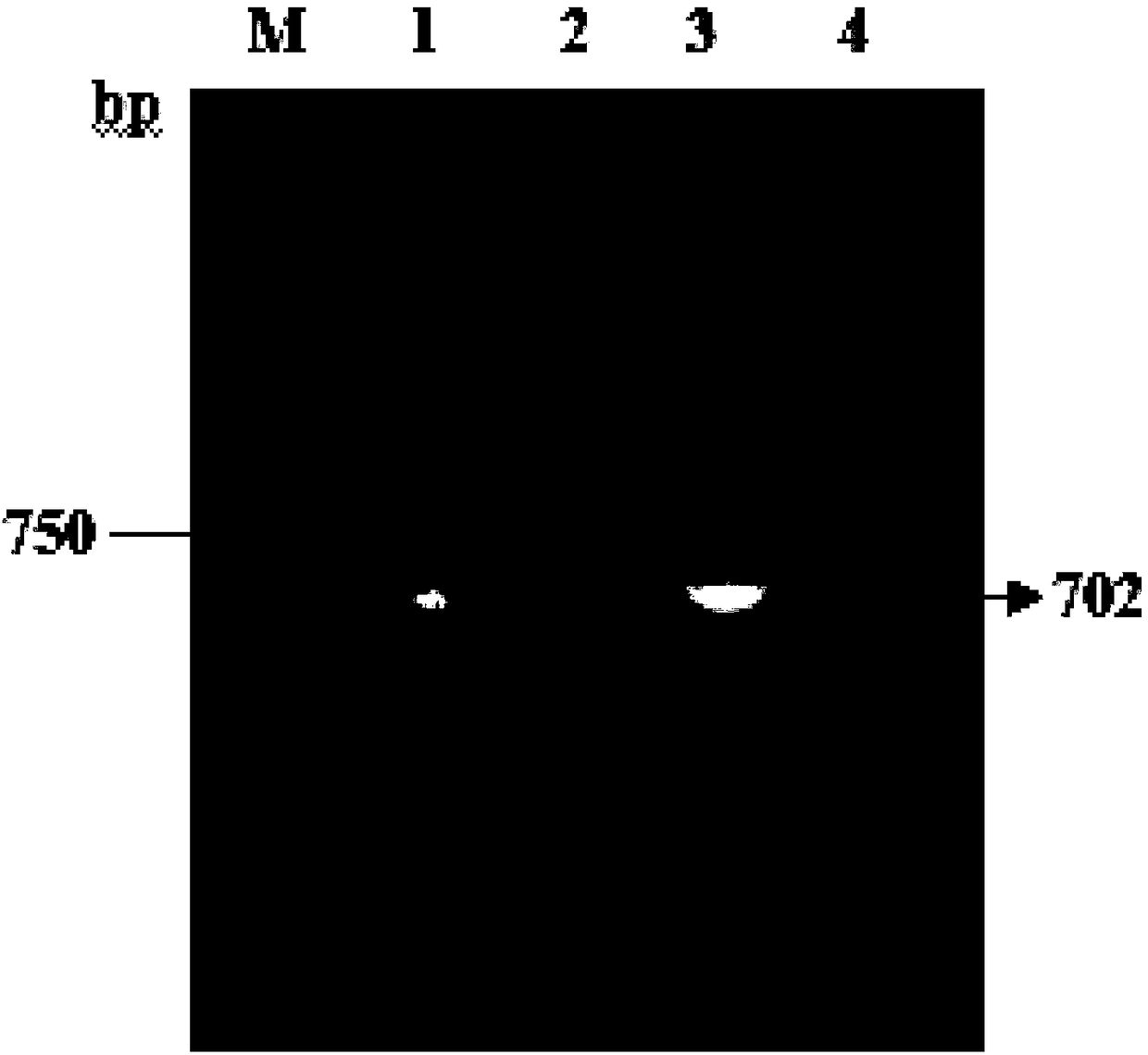
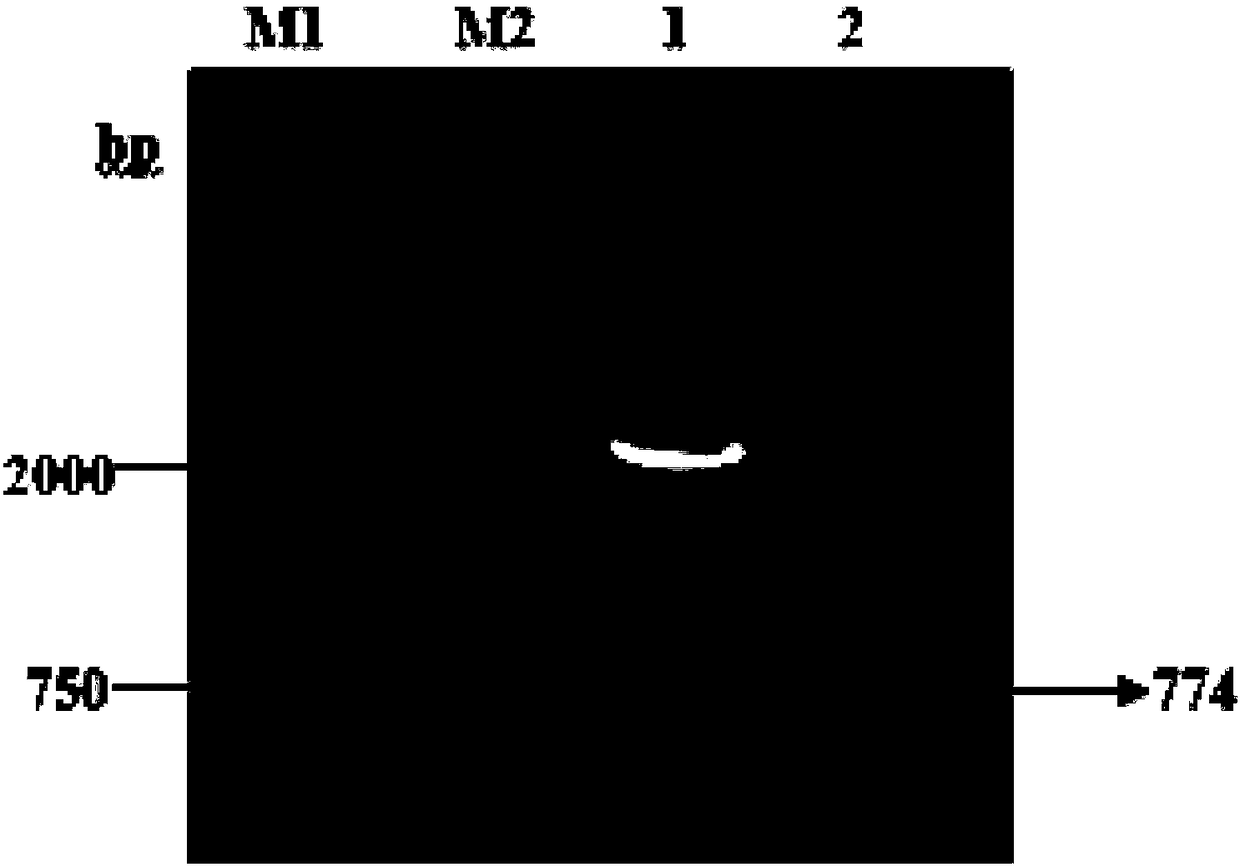
[0044] A preparation method of anti-apoptotic protein PTD-FNK protein, comprising the following steps:
[0045] (1) Take the fresh liver tissue of SD rats, and extract the total RNA according to the instructions of the RNA isolater reagent;
[0046] (2) Construction of the cloning plasmid pUM19-T-Bcl-xL;
[0047] (3) Construct the prokaryotic expression plasmid pET28a(+)-PTD-Bcl-xL;
[0048] (4) Site-directed mutation of the prokaryotic expression plasmid pET28a(+)-PTD-Bcl-xL to obtain the recombinant plasmid pET28a(+)-PTD-FNK plasmid;
[0049] (5) Induce the expression of the fusion protein PTD-FNK in BL21 competent cells, and induce for 14 hours at 30°C;
[0050] (6) Collect bacteria, ultrasonically crush, power 400W, crush for 30min, sonicate for 2S, pause for 6S as a cycle, centrifuge for the first time, 12000rpm, 4°C, centrifuge for 20min, collect supernatant, and use inclusion body washing solution for precipitation ( 10mmol / L Tris-HCL, 50mmol / L NaCl, 2mol / L urea) twi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com