Application of heat shock protein 20 in oxidative stress injury diseases
A technology of heat shock protein and oxidative stress, which is applied in the field of application of heat shock protein 20 in oxidative stress injury diseases, can solve problems that have not been reported, and achieve prevention of oxidative stress injury, high clinical application prospects, delay Effects of disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
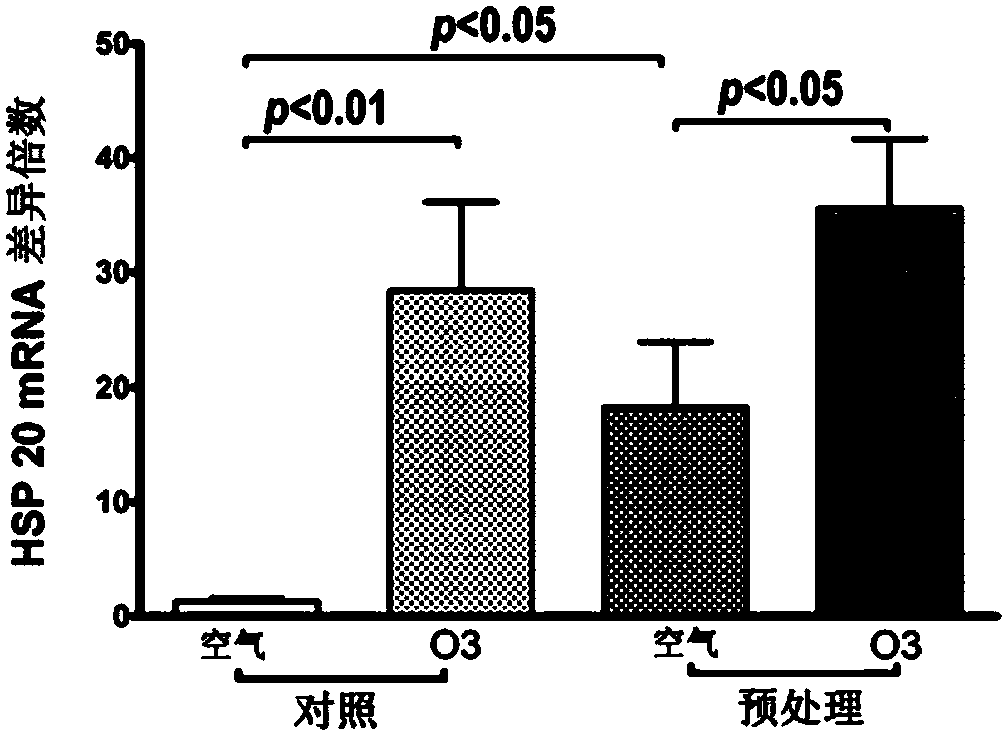
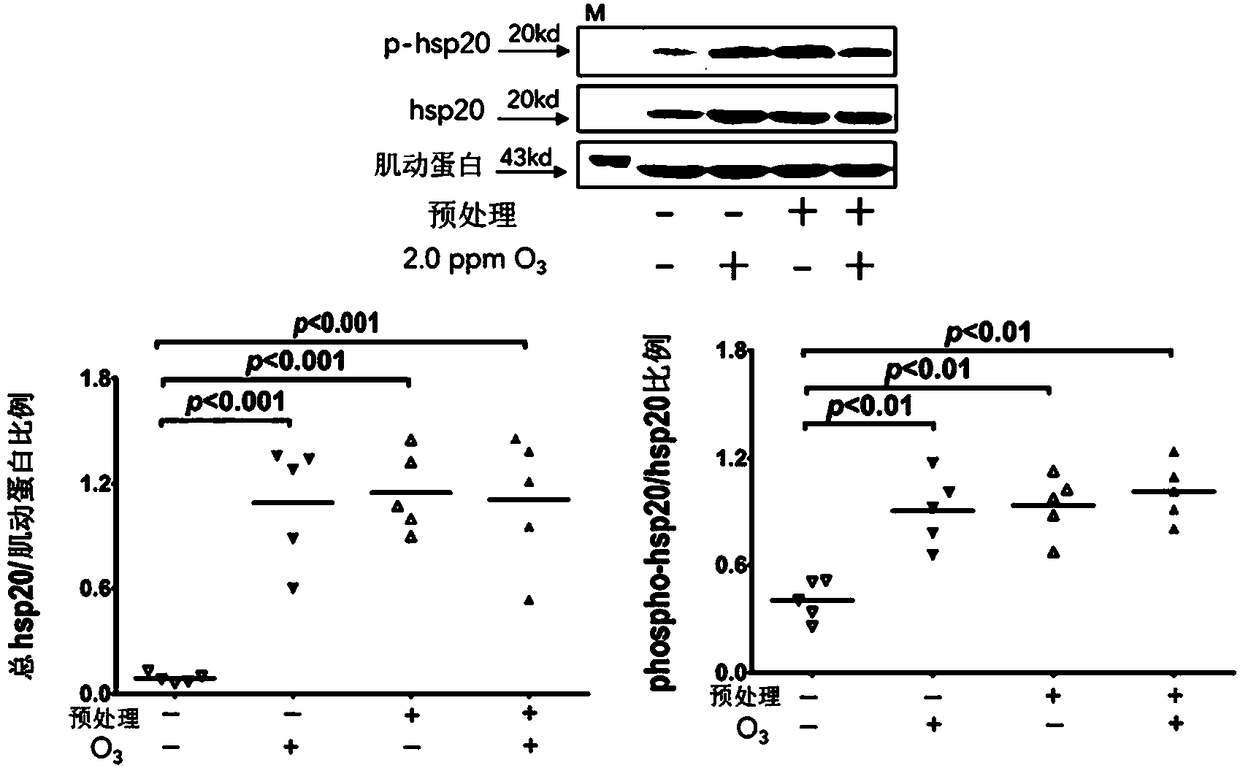
[0048] Embodiment 1: animal experiments
[0049] 1. Materials and methods
[0050] 1. Animal grouping and model preparation
[0051]BALB / c mice: special pathogen-free grade, female, 8-10 weeks old, weighing about 20 g, purchased from Shanghai Shrek Experimental Animal Center. All mice were kept in the independent ventilation system (IVC) of the Animal Experiment Center of the First People's Hospital Affiliated to Shanghai Jiaotong University, with an ambient temperature of 22°C-24°C, a humidity of 40-60%, 12-hour light control, and free access to food and water. All operations and experimental procedures were approved by the Experimental Animal Ethics Committee of the First People's Hospital Affiliated to Shanghai Jiaotong University.
[0052] 20 animals were randomly divided into 4 groups, 5 animals in each group:
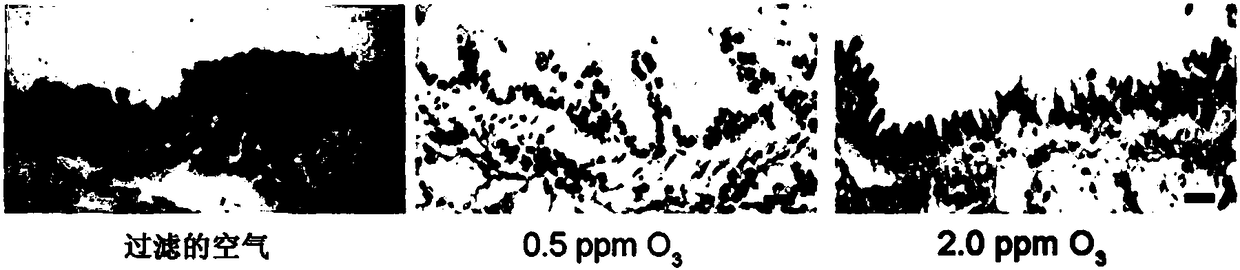
[0053] Normal + air exposure group: exposed to filtered air on the 1st, 3rd, 5th, and 7th days, each time for 1 hour, and exposed to filtered air for 3 hours o...
Embodiment 2
[0080] Example 2: Cell experiment
[0081] 1. Materials and methods
[0082] 1. Cells
[0083]Human airway epithelial cell line BEAS-2B cells (purchased from ATCC) were cultured in vitro. RPMI1640 culture medium containing 10% fetal bovine serum was used.
[0084] 2. Determine H 2 o 2 Stimulus concentration and observation time
[0085] Cells were incubated in a 24-well plate, given gradually increasing H 2 o 2 Concentrations (0.1, 1.0, 10, 100, μM) were stimulated for 1 hour, and the cell viability was detected by MTT at 0, 12 and 24 hours respectively, and the expression of HSP20 protein and gene level were detected by Western blot and RT-qPCR.
[0086] 3. Functional experiment of HSP20 and its phosphorylated protein
[0087] The cells were transfected with pEX-3-HSP20 (wild type overexpression vector), pEX-3-HSP20(S16A) (non-phosphorylated overexpression vector) and pEX-3 (empty vector). Refer to the specific process 2000 (purchased from ThermoFisher Scientific C...
Embodiment 3
[0098] Embodiment 3: Molecular biology detection
[0099] 1. Western blot detection
[0100] After weighing the lung tissue, add the lysate according to the volume of 1:10, then carry out shear dispersion and emulsification on ice, and centrifuge the lung tissue homogenate (or cell lysate) at 13,000 rpm for 15 minutes at 4°C; take 100 μl of the supernatant and add it to the After the sample buffer was boiled at 95°C for 15 minutes, centrifuged slightly and placed at -80°C for testing, another 5 μl of the homogenate supernatant was used to detect the protein concentration by the BCA method. Protein electrophoresis was performed using 10% SDS-PAGE. Load 75 μg of each sample, and determine the loading volume according to the protein concentration. 80V voltage for 30 minutes, 120V voltage for 90 minutes, semi-dry electrotransfer membrane, 20V for 7 minutes. After the transfer was completed, the transfer efficiency was judged by Ponceau red pre-staining. After rinsing with 1×TBS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com