1-(8-arylnaphthyl)phosphine ligand and preparation method thereof as well as phosphine-gold compound and application
A technology of phosphine ligands and complexes, applied in the field of organic synthesis, can solve problems such as harsh conditions, narrow substrate application range, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0034] According to a preferred embodiment of the present invention, the substituted or unsubstituted heteroaryl is selected from R 1 and R' are each independently selected from methyl, methoxy or trifluoromethyl. Further preferably, R 1 is methoxy; R' is selected from methyl or trifluoromethyl.
[0035] According to a preferred embodiment of the present invention, the unsubstituted aryl is selected from phenyl,
[0036]
[0037] According to a preferred embodiment of the present invention, the ligand is represented by the following formula, wherein Ph represents phenyl,
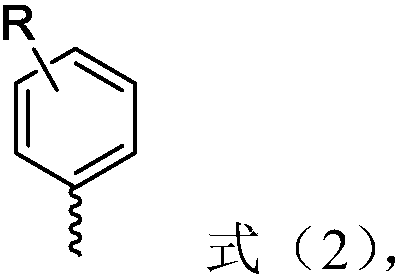
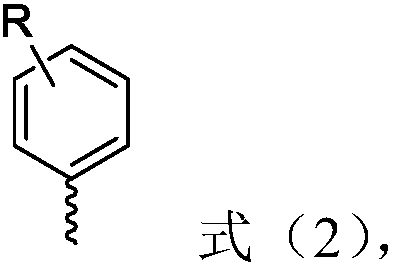
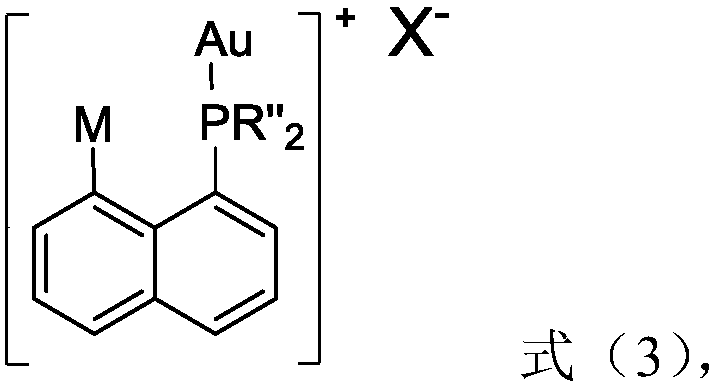
[0038] Among them, R is selected from H, 3-methoxy, 4-methyl, 4-Cl, 4-COPh (Ph represents phenyl), 4-CF 3 , 4-9H-carbazole, 2 R" each independently selected from phenyl or cyclohexyl;
[0039]
[0040] R' is selected from methyl or trifluoromethyl;
[0041]
[0042] The second aspect of the present invention provides a method for preparing a 1-(8-arylnaphthyl)phosphine ligand, the method ...
Embodiment 1
[0079] This example is used to illustrate the preparation of 1-(8-phenylnaphthyl)diphenylphosphine.
[0080] 1-naphthyldiphenylphosphine (1mmol), bromobenzene (1mmol), [Rh(cod)Cl] 2 (0.025mmol), lithium tert-butoxide (3mmol) and toluene (5mL) were mixed in a pressure tube, heated to 110°C under nitrogen protection, and reacted at this temperature for 24 hours. After cooling to room temperature, the target product was separated by silica gel column chromatography (the volume ratio of petroleum ether to dichloromethane was 1:20) The yield was 82%. This product is analyzed, and the results are as follows:
[0081] 1 H NMR (400MHz, CDCl 3 ):δ7.91–7.88(m,2H,Ar),7.48(t,J=7.5Hz,1H,Ar),7.36–7.29(m,2H,Ar),7.22–7.15(m,8H,Ar) ,7.09–7.04(m,4H,Ar),6.94(t,J=7.2Hz,4H,Ar). 13 CNMR (101MHz, CDCl 3):δ143.5(d,J C-P =5.1Hz,Ar),141.3(d,J C-P =3.6Hz,Ar),139.4(d,J C-P =16.7Hz,Ar),137.2(s,Ar),135.5(s,Ar),135.2(d,J C-P =10.1Hz,Ar),134.9(d,J C-P =4.3Hz,Ar),133.7(d,J C-P =20.7Hz,Ar),130.8(...
Embodiment 2
[0083] This example is used to illustrate the preparation of 1-(8-p-methylphenylnaphthyl)diphenylphosphine.
[0084] 1-Naphthyldiphenylphosphine (1mmol), p-methylbromobenzene (1mmol), [Rh(cod)Cl] 2 (0.025mmol), lithium tert-butoxide (3mmol) and toluene (5mL) were mixed in a pressure tube, heated to 120°C under nitrogen protection, and reacted at this temperature for 24 hours. After cooling to room temperature, the target product was separated by silica gel column chromatography (the volume ratio of petroleum ether to dichloromethane was 1:20) The yield was 84%. This product is carried out nuclear magnetic resonance analysis, the result is as follows:
[0085] 1 H NMR (400MHz, CDCl 3 ): δ7.87(t, J=7.4Hz, 2H, Ar), 7.46(t, J=7.6Hz, 1H, Ar), 7.31(dd, J=15.4, 7.3Hz, 2H, Ar), 7.22– 7.15(m,7H,Ar),6.96–6.92(m,6H,Ar),6.84(d,J=7.7Hz,2H,Ar),2.33(s,3H,CH 3 ). 13 C NMR (101MHz, CDCl 3 ):δ141.4(d,J C-P =3.7Hz,Ar),140.4(d,J C-P =5.1Hz,Ar),139.6(s,Ar),139.4(s,Ar),137.3(s,Ar),136.5(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com