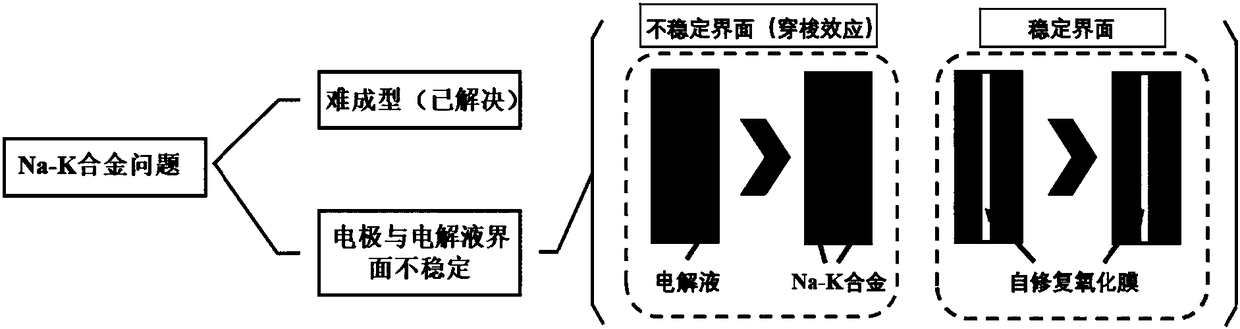
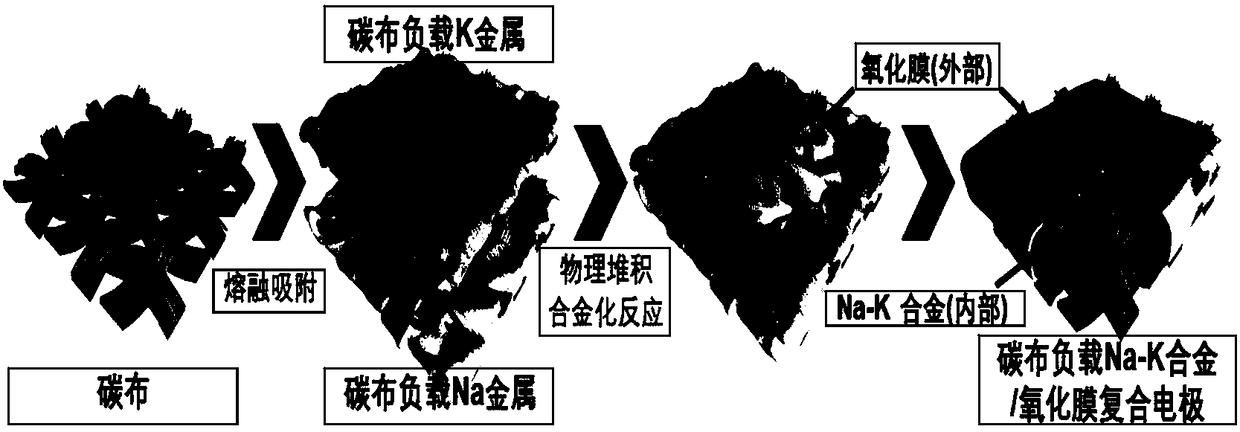
Self-repairing oxidization film-coated Na-K liquid-state alloy electrode and preparation method and application thereof
A liquid alloy and oxide film technology, applied in battery electrodes, electrode carriers/current collectors, circuits, etc., can solve Na-K liquid alloy shedding, without any solution strategy, and does not aim at stabilizing Na-K liquid alloy electrodes and electrolysis Liquid and other problems, to achieve high Coulombic efficiency, improve energy density and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Heat 0.1g of K metal and 0.028g of Na metal in a glove box to 450°C and melt respectively, and then use tweezers to contact carbon cloth (thickness 2mm) with a length of 1cm with the two molten metals, and take them out after they are completely absorbed. Cool to room temperature 25°C. Stack the carbon cloth loaded with K metal and Na metal respectively, and the alloying reaction of the two metals occurs on the surface of the carbon cloth, and a self-healing oxide film is formed on the surface. After a period of reaction, a self-healing oxide film is formed to cover Na-K Liquid alloy electrodes.
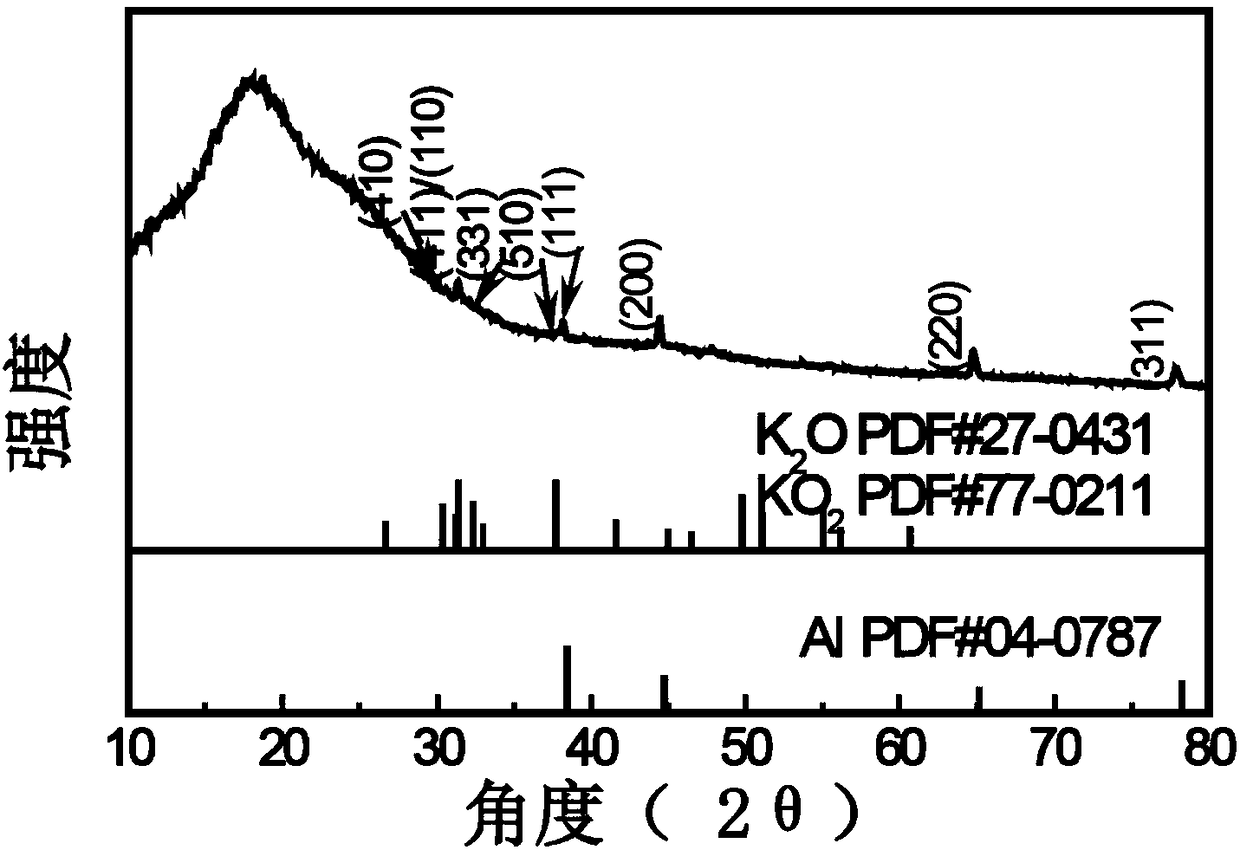
[0047] The XRD diffractogram of the self-repairing oxide film coating Na-K liquid alloy electrode surface that makes in embodiment 1 is as follows image 3 shown. As shown in the figure, the prepared electrode has no characteristic peaks of Na metal and K metal, indicating that the formed alloy is a liquid Na-K alloy. Also found weak KO 2 and K 2 The peak of O indicates t...
Embodiment 2
[0049] Adjust the oxygen content in the glove box to 0.2ppm, and the nitrogen content to 0.1ppm. Heat 0.2g of K metal and 0.056g of Na metal in a glove box to 400°C to melt respectively, and then use tweezers to contact the two kinds of molten metal with a carbon cloth (thickness 2mm) of 1cm in length and width. Cool to room temperature 25°C. Stack the carbon cloth loaded with K metal and Na metal respectively, and the alloying reaction of the two metals occurs on the surface of the carbon cloth, and a self-healing oxide film is formed on the surface. After a period of reaction, a self-healing oxide film is formed to cover Na-K Liquid alloy electrodes.
[0050] The XRD diffraction pattern that obtains electrode is similar to embodiment 1, finds in addition a small amount of KN 3 peak.
Embodiment 3
[0052] Heat 0.2g of K metal and 0.056g of Na metal in a glove box to 400°C to melt respectively, and then use tweezers to contact the two kinds of molten metal with a carbon cloth (thickness 2mm) of 1cm in length and width. Cool to room temperature 25°C. Immerse the carbon cloth loaded with K metal and Na metal respectively in the electrolyte (the solute is KPF with a molar ratio of 1:1 6 and NaPF 6 ; The organic solvent is a solution composed of ethylene carbonate (EC) and dimethyl carbonate (DMC) at a volume ratio of 1:1, KPF 6 and NaPF 6 The concentration in the electrolyte is 1mol / L), and stacked, the alloying reaction of the two metals occurs on the surface of the carbon cloth, and a self-repairing oxide film with new components is formed on the surface. After a period of reaction, a self-repairing oxide film is formed. The oxide film covers the Na-K liquid alloy electrode.
[0053] The obtained XRD diffraction pattern of the electrode is similar to that of Example 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com