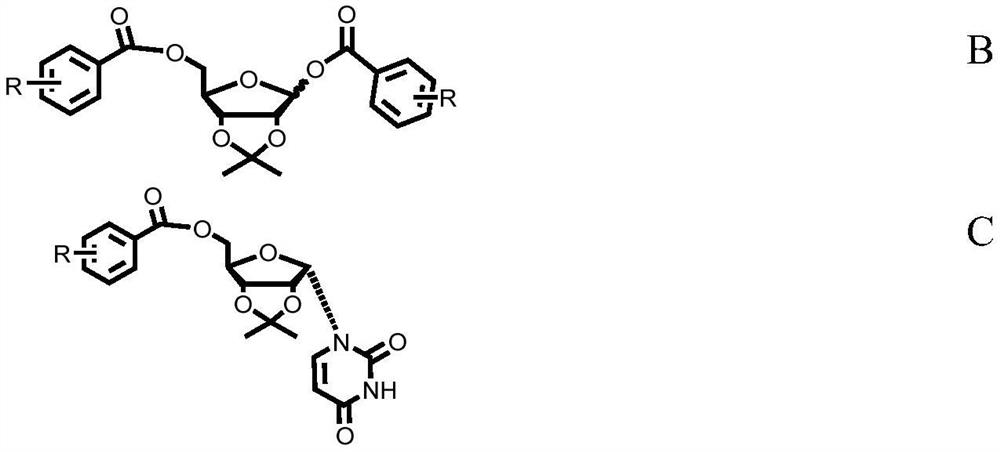
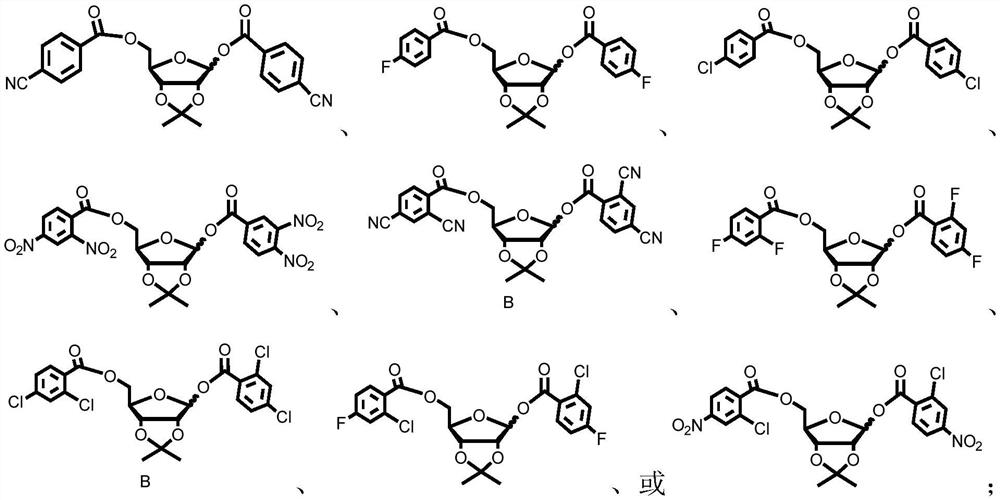
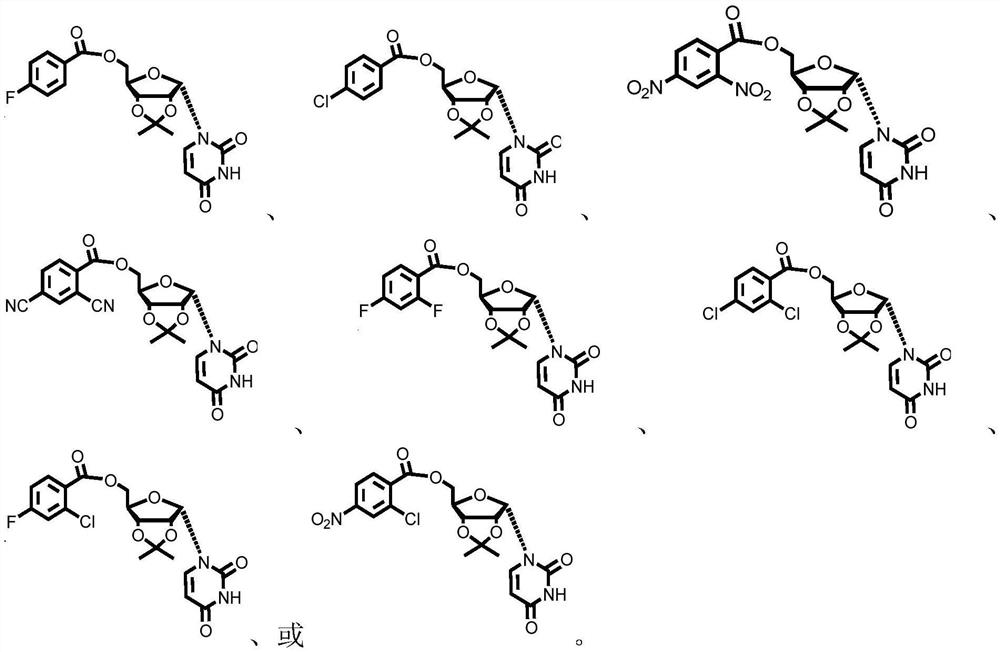
Preparation method of α-uridine nucleoside
A technology of uracil and compounds, applied in the field of nucleoside compound synthesis, which can solve problems such as industrialization resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Preparation of α-uridine nucleoside (method one, R=NO 2 , located in the para position of the benzoyl group)
[0087] 1. Preparation of 2,6-O-di-p-nitrobenzoyl-3,4-O-acetonylidene-D-ribose:
[0088]
[0089] Under the protection of argon, put 100g of D-ribose into a dry 1L three-necked reaction flask, add 500ml of acetone to stir and suspend; Cool the system down to 0±3°C, add 75.6g of 2,2-dimethoxypropane dropwise to the reaction solution, and control the temperature at 0±3°C; return the reaction solution to 15±3°C and stir for reaction, after 2 hours of reaction, TLC The plate showed essentially no starting material and the reaction was considered complete; then 4.8 g NaHCO was added 3 After stirring for half an hour, suction filtration was performed, and the filtrate was concentrated to dryness under reduced pressure at 40±5°C to obtain crude IM-1, which was directly used in the next step without purification. Add 800ml of dichloromethane to the concentrate to ...
Embodiment 2
[0099] Preparation of α-uridine nucleoside (R=CN, located in the para position of benzoyl)
[0100] 1. Preparation of 2,6-O-di-p-cyanobenzoyl-3,4-O-acetonylidene-D-ribose:
[0101]
[0102]The preparation of IM-1 was as described above, and 100 g of D-ribose was fed for reaction preparation to obtain the crude product of IM-1. This crude product was dissolved in 800ml of dichloromethane, and added under stirring, 201.8g of triethylamine, and 275.7g of p-cyanobenzoyl chloride were slowly added. After stirring and reacting at room temperature for 2.5 hours, sample TLC plate analysis, IM-1 is not obvious, and the reaction is considered to be complete; after the reaction is completed, as described above, after extraction and concentration to dryness, the crude product of compound B is obtained without purification, and the same compound B It is a mixture of α and β configurations.
[0103] 2. Preparation of 2'3'-O-acetonylidene-α-uridine:
[0104]
[0105] Add 50g of Urac...
Embodiment 3
[0111] Preparation of α-uridine nucleoside (R = F, located in the para position of benzoyl)
[0112] 1. Preparation of 2,6-O-di-p-fluorobenzoyl-3,4-O-acetonylidene-D-ribose:
[0113]
[0114] The preparation of IM-1 was as described above, and 100 g of D-ribose was fed for reaction preparation to obtain the crude product of IM-1. This crude product was dissolved in 800ml of dichloromethane, and added under stirring, 201.8g of triethylamine, and 264g of p-fluorobenzoyl chloride were slowly added. After stirring and reacting at room temperature for 1.5 hours, sample TLC plate analysis, IM-1 is not obvious, and the reaction is considered to be complete; after the reaction is completed, as described above, after extraction and concentration to dryness, the crude product of compound B is obtained without purification, and the same compound B It is a mixture of α and β configurations.
[0115] 2. Preparation of 2'3'-O-acetonylidene-α-uridine:
[0116]
[0117] Add 50g of Ur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com