A kind of renaturation method of ribonuclease inhibitor inclusion body
A technology of ribonuclease and inclusion body, which is applied in the fields of chemical and biological engineering, can solve the problems of incomplete removal of TrxA label, failure to effectively use inclusion body, and low renaturation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
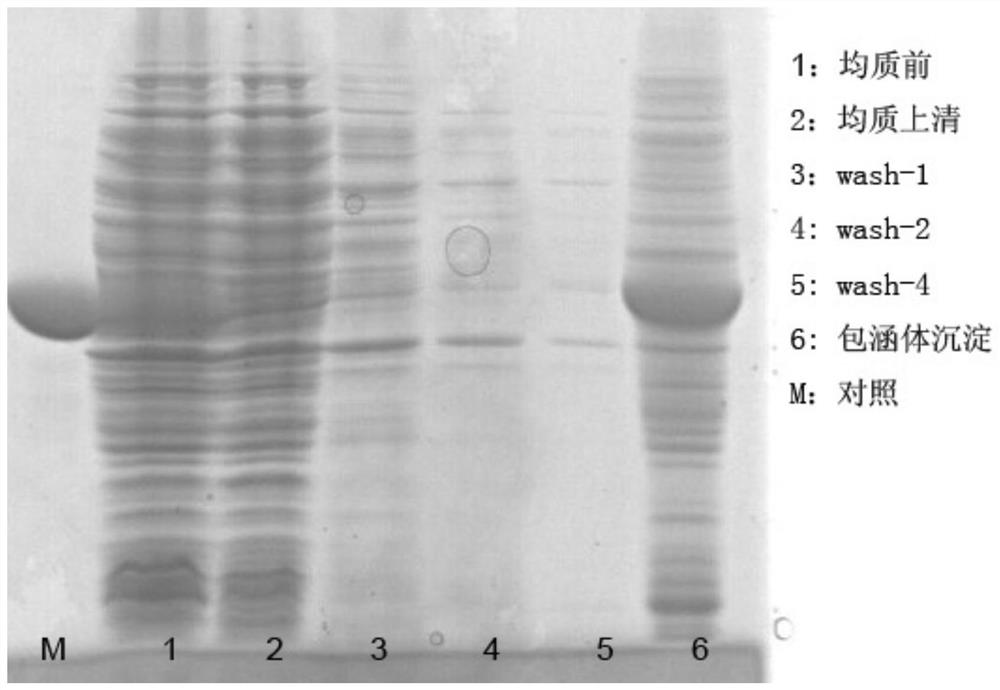
[0072]Embodiment 1, the purification of RI inclusion body
[0073] (1) Construction of ribonuclease inhibitor RI carrier
[0074] Extract total RNA from discarded placental tissue isolated from human body, then perform RT-PCR to amplify the RI gene sequence (adding 6×His-tag at the N-terminal), recover by enzyme digestion, connect to pET28b vector, and transform into DH5a competent Cells were selected for single clone culture and the extracted plasmid (pET28b-6*His-RI) was transformed into the expression host bacteria.
[0075] (2) Cell preparation
[0076] The constructed BL21DE3 expression strain was inoculated into 100ml LB medium (Kana + ), 37°C, 230rpm, 16hr. Transfer the seed liquid to 2×YT medium according to the inoculation amount of 1%, and carry out fermentation culture. When the OD600 of the bacterial liquid reaches 0.9, add the inducer IPTG to the final concentration of 0.2mM, induce for 4.5 hours, and collect the bacterial cells by centrifugation after the indu...
Embodiment 2
[0084] Embodiment 2, renaturation of RI inclusion body
[0085] (1) Denaturation and dissolution of inclusion bodies
[0086] Take 1 g of the purified inclusion body, add it to 10 ml of resuspension buffer, and stir rapidly for 1 hr, then add the above resuspension into 10 ml of inclusion body denaturation solution stored at 4°C, and stir rapidly for 30 min, at which time the inclusion body is completely dissolved, and it is Transparent.
[0087] Resuspension buffer formula: DTT 1mM, EDTA 1mM, sodium chloride 50mM, glycerol 10% (v / v), Tris-HCl 15mM, pH 7.5.
[0088] Inclusion body denaturing solution formula: DTT 1mM, denaturant urea 6M, Tris-HCl 35mM, EDTA 1mM, sodium chloride 50mM, glycerol 10% (v / v), pH 7.5.
[0089] (2) Refolding of inclusion bodies
[0090] Quickly pour the above-mentioned denaturing solution into 100ml of the refolding solution stored at 4°C, stir rapidly to mix, and then stir at a low speed for 20 hours to obtain the treated refolding solution.
[0...
Embodiment 3
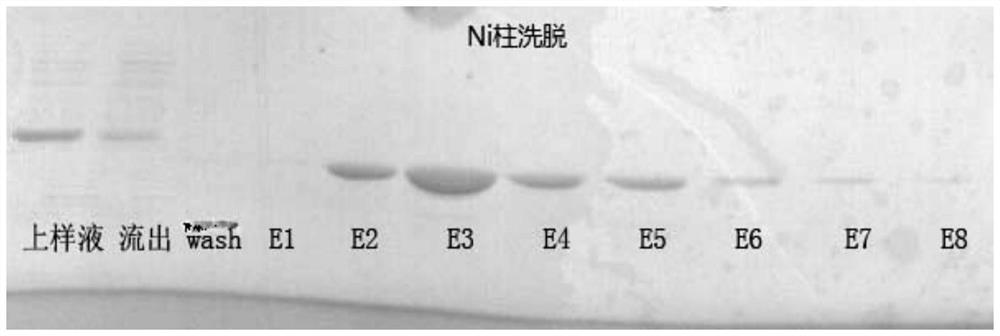
[0093] Example 3, Column Purification of RI Refolding Solution (chromatography at 4°C)
[0094] (1) Sample loading
[0095] Dilute the treated refolding solution obtained above to a protein concentration of 0.5-1 mg / ml, adjust the pH to 8.0, and load the sample into Ni-sepharose FF (10 ml, GE Company) at a flow rate of 2 ml / min.
[0096] (2) Washing to remove RNase A
[0097] After loading the sample, first wash 2 times the column volume with the column equilibration buffer, then wash the column with the washing buffer at a flow rate of 2ml / min, and perform SDS-PAGE electrophoresis at the same time to detect the banding of the washing solution. When the RNase A band appears, stop washing.
[0098] Column equilibration buffer formula: DTT 1mM, EDTA 1mM, sodium chloride 50mM, glycerol 10% (v / v), Tris-HCl 35mM, pH 7.5.
[0099] Washing buffer formulation: DTT 1 mM, EDTA 1 mM, sodium chloride 300 mM, NaAC / HAC 35 mM, pH 5.0, glycerol 5% (v / v).
[0100] (3) Gradient elution of R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com