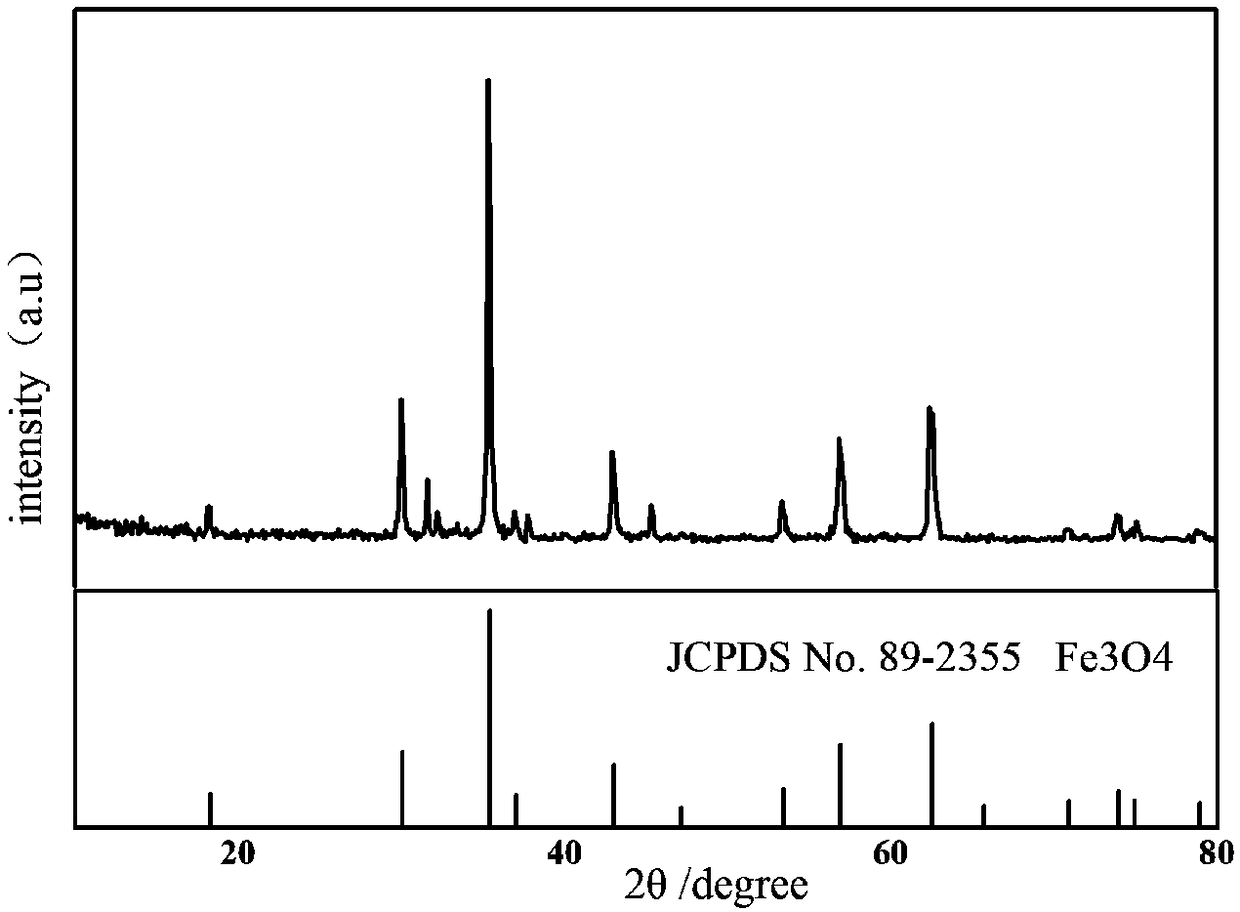
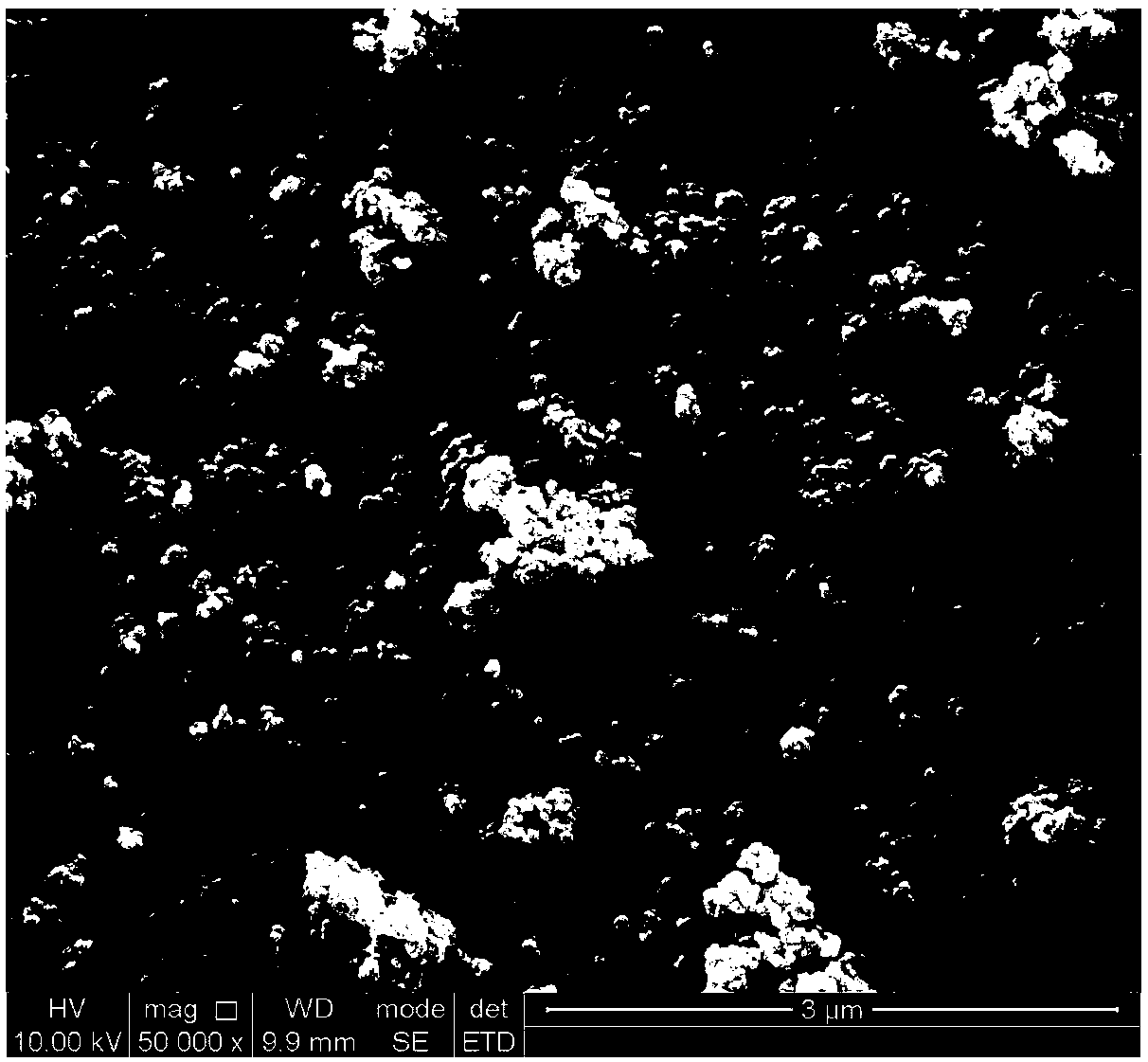
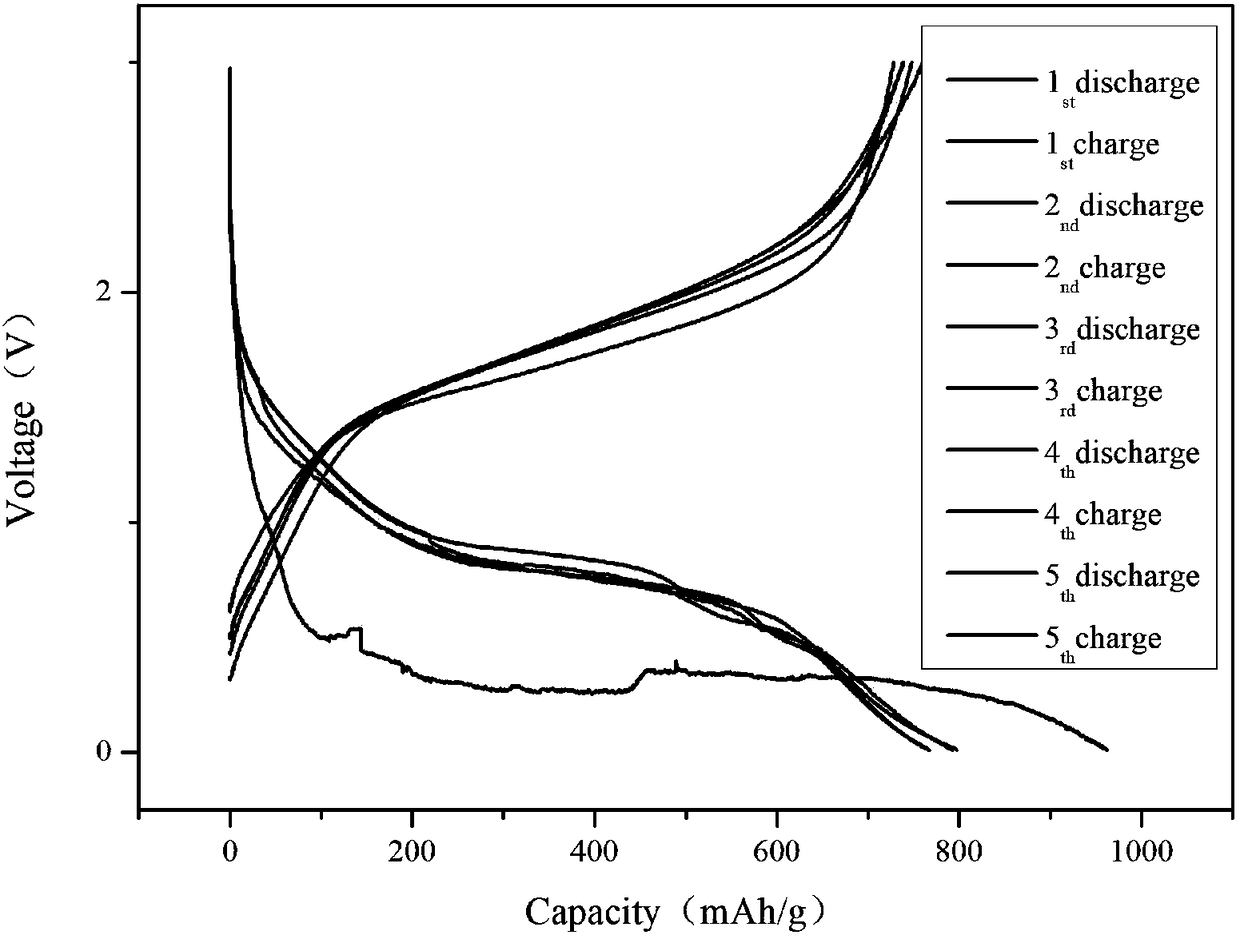
In-situ nano Fe3O4@C composite porous lithium ion battery anode material and preparation method thereof
A lithium-ion battery and in-situ composite technology, applied in battery electrodes, nanotechnology, nanotechnology, etc., can solve the problems of inability to balance performance and cost requirements, poor rate performance and cycle stability, and high requirements for the synthesis process. Simple and controllable, improved electrochemical stability, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] with Fe 3+ : C 6 h 5 o 7 3- Molar ratio 3:2 Weigh FeCl 3 and Na 3 C 6 h 8 o 7 2H 2 O was dissolved in deionized water to prepare FeCl with a concentration of 0.5mol / L 3 solution, and 1.2 mol / L Na 3 C 6 h 8 o 7 solution, the two are mixed uniformly to obtain a mixed solution. Then add 30 mL of absolute ethanol to the mixed solution of the two, and a reddish-brown precipitate is found, and continue to place the mixed solution with added absolute ethanol in a constant temperature water bath at 60°C for 30 min. Subsequently, the reaction precipitate was centrifuged, washed three times with absolute ethanol / deionized water, and dried by vacuum freeze-drying to obtain the ferric citrate precursor. Then place the ferric citrate precursor in a horizontal tube furnace, heat up to the carbonization temperature of 600 ºC at a rate of 5 ºC / min under argon gas, and cool it down to room temperature after 3 hours of holding time, and the final carbonization temperature ...
Embodiment 2
[0027] with Fe 3+ : C 6 h 5 o 7 3- Molar ratio 1.5:1 Weigh FeCl 3 and Na 3 C 6 h 8 o 7 2H 2 O was dissolved in deionized water to prepare FeCl with a concentration of 0.5mol / L 3 solution, and 1.2 mol / L Na 3 C 6 h 8 o 7 solution, the two are mixed uniformly to obtain a mixed solution. Then add 30mL of absolute ethanol to the mixed solution of the two, and a reddish-brown precipitate was found, and the mixed solution with added absolute ethanol was placed in a constant temperature water bath at 60°C and continuously stirred for 4h. Subsequently, the reaction precipitate was centrifuged, washed three times with absolute ethanol / deionized water, and dried by vacuum freeze-drying to obtain the ferric citrate precursor. Then place the ferric citrate precursor in a horizontal tube furnace, heat up to the carbonization temperature of 600 ºC at a rate of 5 ºC / min under argon gas, and cool it down to room temperature after holding for 1 hour. The final carbonization tempe...
Embodiment 3
[0031] with Fe 3+ : C 6 h 5 o 7 3- Molar ratio 1.8:1 weigh FeCl 3 and Na 3 C 6 h 8 o 7 2H 2 O was dissolved in deionized water to prepare FeCl with a concentration of 0.5mol / L 3 solution, and 1.2 mol / L Na 3 C 6 h 8 o 7 solution, the two are mixed uniformly to obtain a mixed solution. Then add 30mL of absolute ethanol to the mixed solution of the two, and a reddish-brown precipitate was found, and the mixed solution with added absolute ethanol was placed in a constant temperature water bath at 60°C and continuously stirred for 4h. Subsequently, the reaction precipitate was centrifuged, washed three times with absolute ethanol / deionized water, and dried by vacuum freeze-drying to obtain the ferric citrate precursor. Then place the ferric citrate precursor in a horizontal tube furnace, heat up to the carbonization temperature of 600 ºC at a rate of 5 ºC / min under argon gas, and cool it down to room temperature after 3 hours of holding time, and the final carbonizat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com