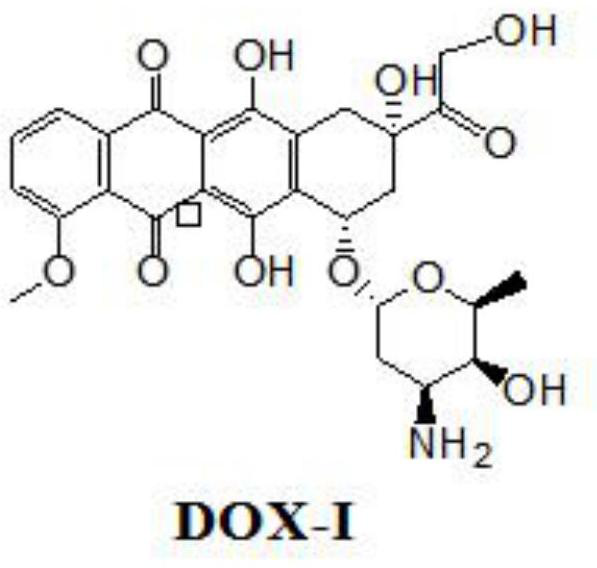
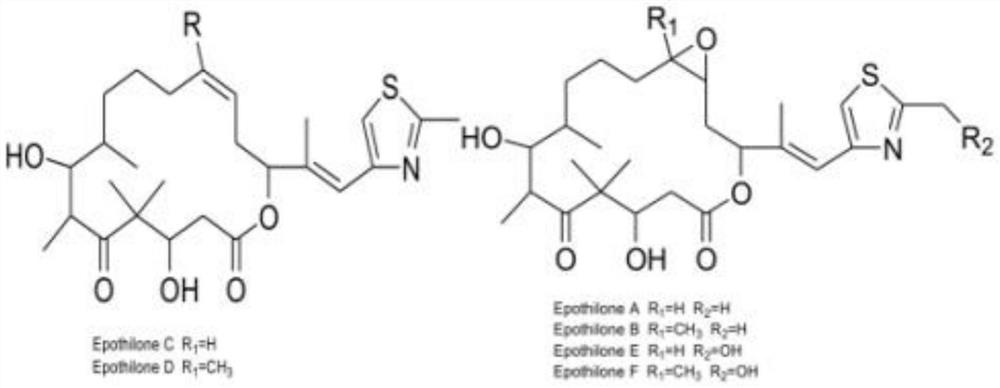
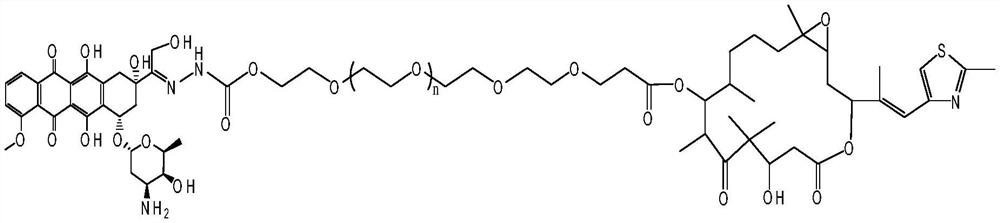
A kind of doxorubicin polyethylene glycol epothilone b conjugate and preparation method thereof
A technology of epothilone and PEGylation, which is applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., to achieve high selectivity and yield, enhance the efficacy of chemotherapy, and reduce the effects of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 50ml three-neck flask equipped with a mechanical stirrer, a thermometer, and a nitrogen bag, add Hz-PEG 22 - COOH (10 mmol), phosphoric acid (1 mmol) and 30 ml DMF. Under the protection of nitrogen, the system was stirred at a room temperature of 25° C. in the dark for 48 hours. Add 100 microliters of triethylamine (TEA), and then use a dialysis membrane to perform dialysis in a phosphate buffer solution of pH 8.0 until the dialysate no longer turns red, take out the dialysate and freeze-dry to obtain the intermediate compound DOX-PEG22 -COOH (90% yield). The synthesis of the product was confirmed by NMR and HPLC simultaneously.
[0032] Weigh Epothilone B (6mmol) and DOX-PEG22-COOH (6mmol) into a 25mL round bottom flask, stir to dissolve. Under nitrogen protection, 5 mL of dimethyl sulfoxide solution in which coupling agent DCC (6 mmol) and catalyst DMAP (12 mmol) were dissolved was slowly added. Ice bath, take it out after reacting for 2h, and continue to sti...
Embodiment 2
[0034] In a 50ml three-neck flask equipped with a mechanical stirrer, a thermometer, and a nitrogen bag, add Hz-PEG 72- COOH (10 mmol), phosphoric acid (1 mmol) and 30 ml DMF. Under the protection of nitrogen, the system was stirred at a room temperature of 25° C. in the dark for 48 hours. Add 100 microliters of triethylamine (TEA), and then use a dialysis membrane to perform dialysis in a phosphate buffer solution of pH 8.0 until the dialysate no longer turns red, take out the dialysate and freeze-dry to obtain the intermediate compound DOX-PEG22 -COOH (88% yield). The synthesis of the product was confirmed by NMR and HPLC simultaneously.
[0035] Weigh about Epothilone B (12mmol) and DOX-PEG72-COOH (6mmol) into a 25mL round bottom flask, stir to dissolve. Under nitrogen protection, 5 mL of dimethyl sulfoxide solution in which coupling agent DCC (6 mmol) and catalyst DMAP (6 mmol) were dissolved was slowly added. Ice bath, take it out after reacting for 2h, and continue t...
Embodiment 3
[0037] In a 50ml three-neck flask equipped with a mechanical stirrer, a thermometer, and a nitrogen bag, add Hz-PEG 120 - COOH (10 mmol), phosphoric acid (1 mmol) and 30 ml DMF. Under the protection of nitrogen, the system was stirred at a room temperature of 25° C. in the dark for 48 hours. Add 100 microliters of triethylamine (TEA), and then use a dialysis membrane to perform dialysis in a phosphate buffer solution of pH 8.0 until the dialysate no longer turns red, take out the dialysate and freeze-dry to obtain the intermediate compound DOX-PEG22 -COOH (90% yield). The synthesis of the product was confirmed by NMR and HPLC simultaneously.
[0038] Weigh about 12mmol of Epothilone B and 6mmol of DOX-PEG120-COOH into a 25mL round bottom flask, stir to dissolve. Under nitrogen protection, 5 mL of dimethyl sulfoxide solution in which coupling agent DCC (12 mmol) and catalyst DMAP (12 mmol) were dissolved was slowly added. Ice bath, take it out after reacting for 2h, and con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com