Application of FoF1-ATPasebeta protein in promotion or reduction of adsorption of cells on apoptotic bodies
1. The technology of fof1-atpase and apoptotic body, which is applied in the fields of application, peptide/protein components, medical preparations containing active ingredients, etc., can solve the problems of failing to control the accumulation of apoptotic bodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1.F o f 1 - Primer design for ATPaseβ
[0063] Transcriptome data from Spodoptera litura After Parasitization by Microplitis bicoloratus.PLoS ONE 9( 10): Select F from e110967.doi:10.1371 / journal.pone.0110967) o f 1-ATPaseβ gene sequence, F o f 1 - ATPaseβ gene sequence (shown as SEQ ID No.5) has a size of 1551 bp and encodes a total of 516 amino acids (SEQ ID No.6). Specific primers were designed using the primer design software Primer Premier 5. upstream primer F o f 1 -ATPase β-F (SEQ ID No.7) and downstream primer F o f 1 - ATPase β-R (SEQ ID No. 8).
[0064] 2. Extraction of total RNA and cDNA synthesis of Spodoptera litura larvae
[0065] In this experiment, the laboratory protocol was used to extract total RNA from Spodoptera litura larvae. The specific steps are as follows:
[0066] 1) Take 10 3-day-old larvae, place them in EP tubes with a small amount of RNAisoTM Plus lysate, mash and grind them thoroughly, continue to add RNAisoTM Plus lysate unt...
Embodiment 2
[0147] Saccharomyces cerevisiae F o f 1 -The genebank accession number of ATPaseβ is AAA34444.1, Caenorhabditis elegans (Caenorhabditis elegans) F o f 1 -The genebank accession number of ATPaseβ is P46561.2, Drosophila melanogaster F o f 1 -The genebank accession number of ATPaseβ is CAA50332.1, Spodoptera litura F o f 1 -The genebank accession number of ATPaseβ is AFY62978.1, Xenopus laevis F o f 1 - The genebank accession number of ATPaseβ is NP_001080126.1, Homo sapiens F o f 1 - The genebank accession number of ATPaseβ is NP_001677.2. For various representative species from low to high F o f 1 The amino acid sequence of -AT Paseβ carries out the sequence comparison of the Walk A module and the Walk B module, see Figure 4 . Depend on Figure 4 It can be seen that F o f 1 -WalkerA and WalkerB of ATPaseβ are highly conserved in various species.
Embodiment 3
[0149] Only replace pIZT-ATPaseβ with pIZT-ATPaseβ(K202-A), the difference between pIZT-ATPaseβ(K202-A) and pIZT-ATPaseβ lies in the expression of F o f 1 -The amino acid at position 202 of ATPaseβ was mutated from the original lysine (K) to alanine (A), that is, the conserved amino acid lysine (K) in the Walker A module was mutated into alanine (A).
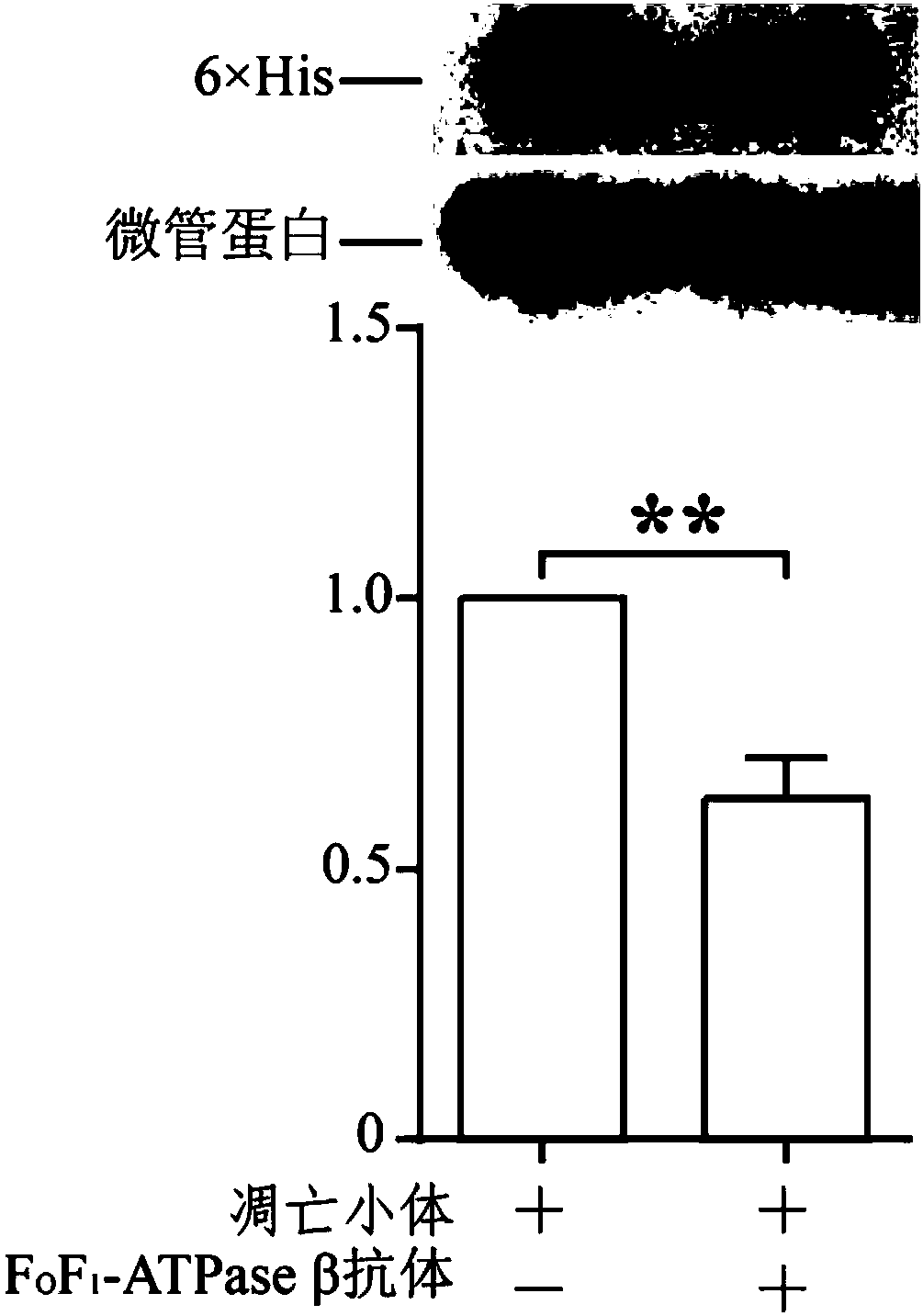
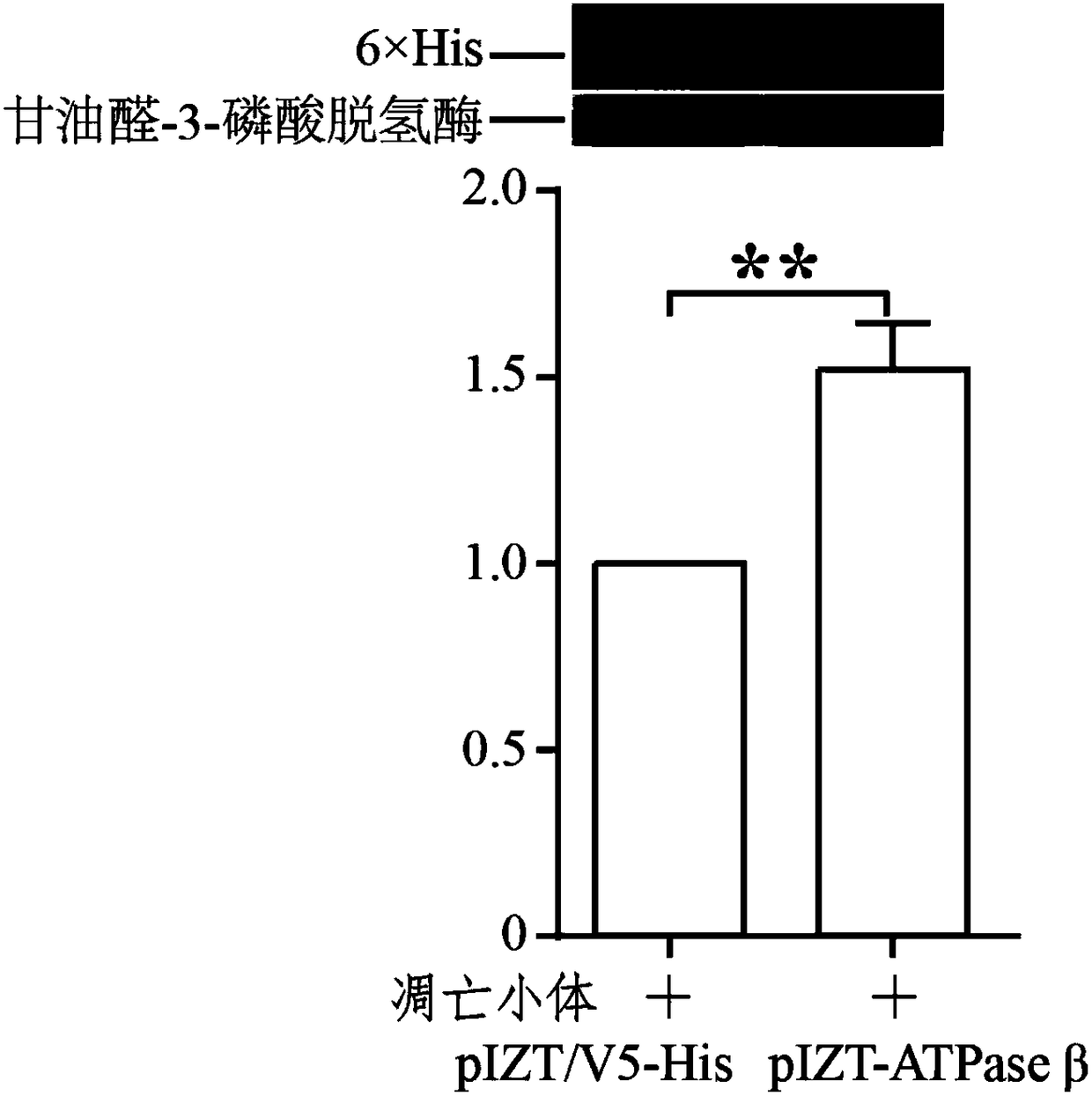
[0150] The operation methods of extraction of total protein, detection of Western bolt, and significant difference analysis are the same as those in Example 1. In this example, glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference.
[0151] see results Figure 5 . Depend on Figure 5 It can be seen that cells overexpressing pIZT-ATPaseβ(K202-A) after mutation have significantly reduced absorption of apoptotic bodies compared with cells overexpressing pIZT-ATPaseβ, indicating that F o f 1 -At least the lysine (K) of Walker A in ATPaseβ plays an important role in the process of apoptotic body uptake.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com