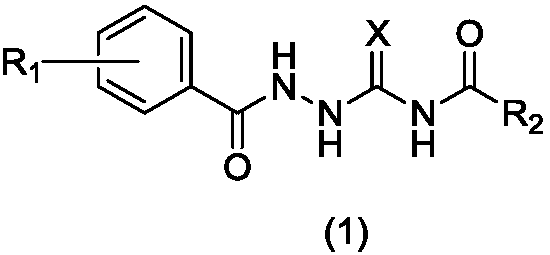
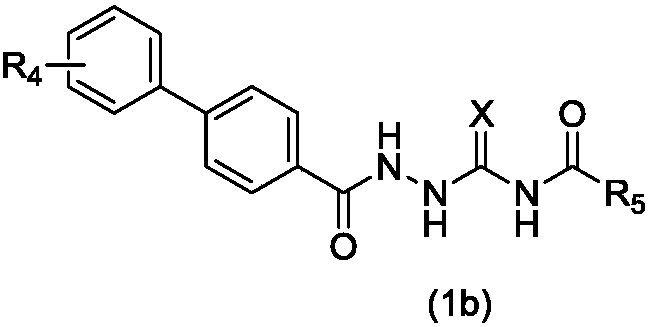
1-substituted benzoyl-4-fatty acyl semicarbazide derivative, preparation method and application thereof as antibacterial agent
A technology of aliphatic acylsemicarbazides and propionyl thiosemicarbazides, which is applied in the field of preparation of 1-substituted benzoyl-4-fatty acylsemicarbazide derivatives, and can solve the problem of 1-aroyl-4-fatty acylaminosulfurides Urea derivatives have little research, weak activity, no antibacterial activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1: Preparation and detection of compound LP-1
[0109]
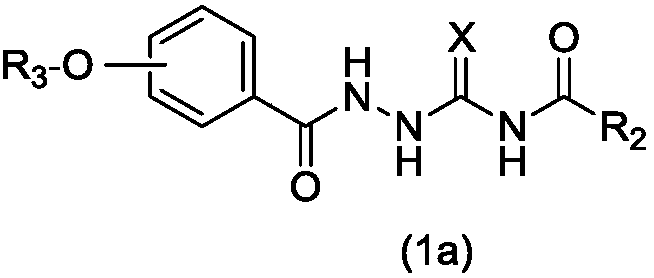
[0110]1.0g (6.5mmol) methyl 4-hydroxybenzoate and 2.7g (19.7mmol) potassium carbonate and 60mL acetone were added to a 100mL three-necked flask, heated to reflux at 60°C, and 1.3ml (9.8mmol) was added thereto After the reaction was completed, the reaction solution was removed under reduced pressure, and 300 mL of water was added to dissolve the product, extracted 3 times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain 1.4 g of methyl 4-pentyloxybenzoate (yield: 99%).
[0111] Add 0.7g (3.1mmol) of methyl 4-pentyloxybenzoate, 5mL of hydrazine hydrate (80%) and 30mL of methanol into a 100mL three-necked flask, heat to reflux at 70°C, and spin the reaction solution to obtain The white solid was washed with water three times and filtered to obtain 0.65 g of 4-pentyloxybenzoic hydrazide (yield: 93%).
[0112] ...
Embodiment 2
[0114] Embodiment 2: Preparation and detection of compound LP-2
[0115]
[0116] 1.0g (5.9mmol) methyl 2,4-dihydroxybenzoate, 2.7g (19.7mmol) potassium carbonate and 60mL acetone were added to a 100mL three-necked flask, heated to reflux at 60°C, and 1.8ml ( 14.5mmol) of 1-bromopentane, after the reaction was completed, the reaction solution was removed under reduced pressure, 300mL of water was added to dissolve the product, extracted 3 times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and spin-dried From the filtrate, 0.7 g of methyl 2-hydroxy-4-pentyloxybenzoate was obtained (yield: 49%).
[0117] Add 0.7g (2.9mmol) methyl 2-hydroxy-4-pentyloxybenzoate, 5mL hydrazine hydrate (80%) and 30mL methanol into a 100mL three-necked flask, heat to reflux at 70°C, spin dry after the reaction is complete In the reaction solution, a white solid was obtained. The white solid was washed three times with water and filtered to f...
Embodiment 3
[0120] Embodiment 3: Preparation and detection of compound LP-3
[0121]
[0122] 1.0g (5.9mmol) methyl 3,4-dihydroxybenzoate, 2.7g (19.7mmol) potassium carbonate and 60mL acetone were added to a 100mL three-necked flask, heated to reflux at 60°C, and 1.8ml ( 14.5mmol) of 1-bromopentane, after the reaction was completed, the reaction solution was removed under reduced pressure, 300mL of water was added to dissolve the product, extracted 3 times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and spin-dried From the filtrate, 0.7 g of methyl 3-hydroxy-4-pentyloxybenzoate was obtained (yield: 49%).
[0123] Add 0.7g (2.9mmol) methyl 3-hydroxy-4-pentyloxybenzoate, 5mL hydrazine hydrate (80%) and 30mL methanol into a 100mL three-necked flask, heat to reflux at 70°C, spin dry after the reaction is complete In the reaction solution, a white solid was obtained, which was washed three times with water and filtered to finally obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com