Preparation method and application of tripterine nanomedicine coated with hyaluronic acid
A technology of tripterine and hyaluronic acid, which is applied in the field of preparation of tripterine nano-drugs, can solve the limitations of the wide application of nano-drug loading technology, complex carrier synthesis process and drug-loading steps, and nano-carrier-assisted drug delivery system There are no problems such as therapeutic effects, and the effects of high-efficiency tumor killing, targeting, and simple and easy preparation methods are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
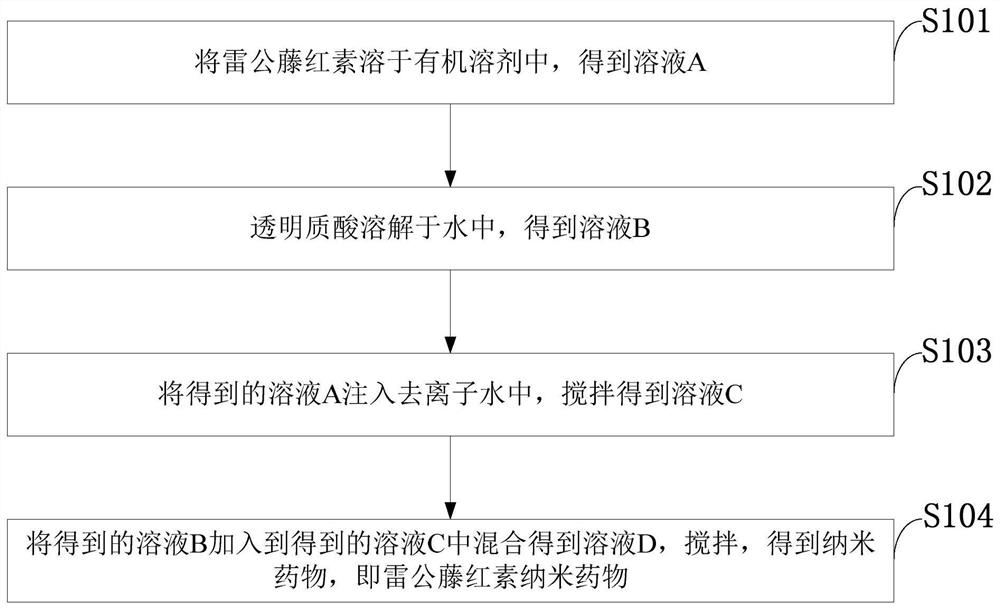
[0035] Such as figure 1 As shown, the preparation method of hyaluronic acid-coated tripterine nanomedicine provided by the embodiments of the present invention comprises the following steps:
[0036] S101: dissolving tripterine in an organic solvent to obtain a solution A;
[0037] S102: the hyaluronic acid is dissolved in water to obtain a solution B;
[0038] S103: inject the obtained solution A into deionized water, and stir to obtain a solution C;
[0039] S104: Add the obtained solution B into the obtained solution C and mix to obtain a solution D, and stir to obtain a nano-medicine, ie tripterine nano-medicine.
[0040] The present invention prepares nano-scale drugs through the precipitation method, which does not need to be used in combination with drug carriers, avoiding the problems of biological system toxicity and immunogenicity caused by carrier materials, and the present invention uses the precipitation method to prepare nano-medicines without nano-carriers, It...
Embodiment 1
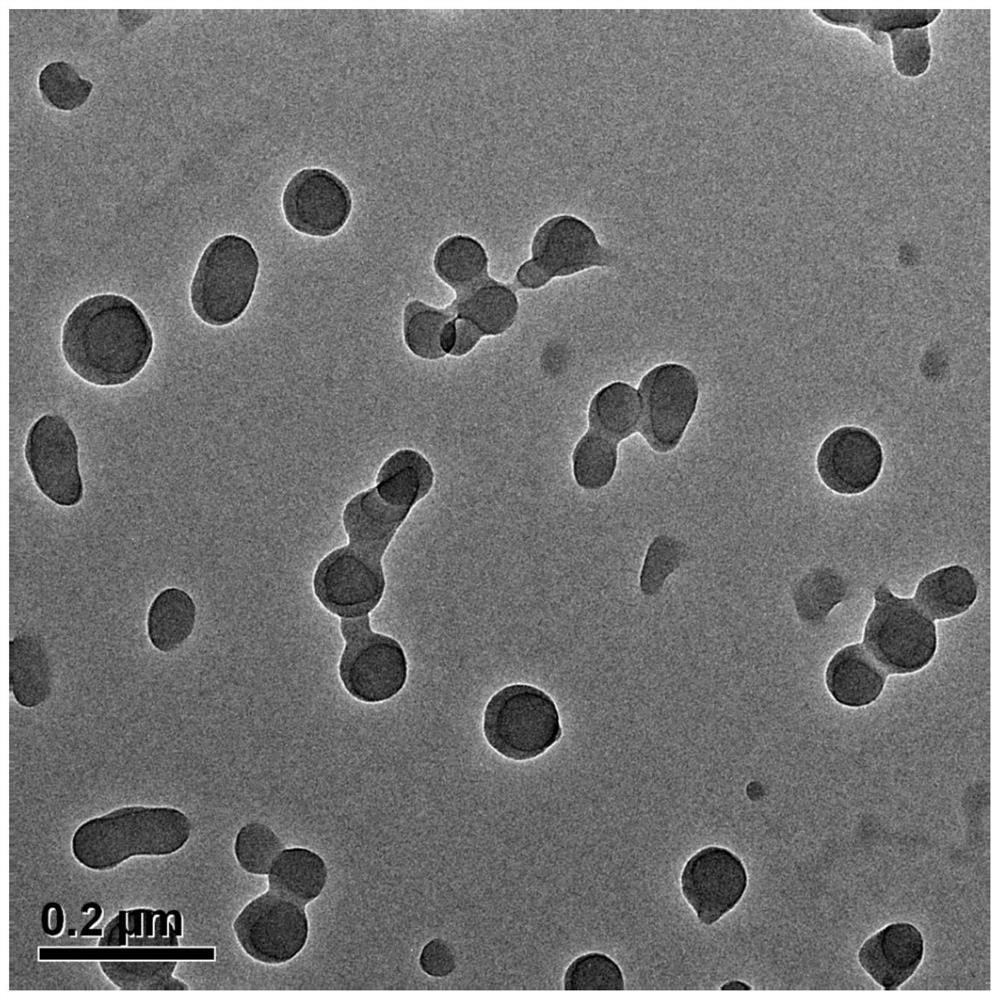
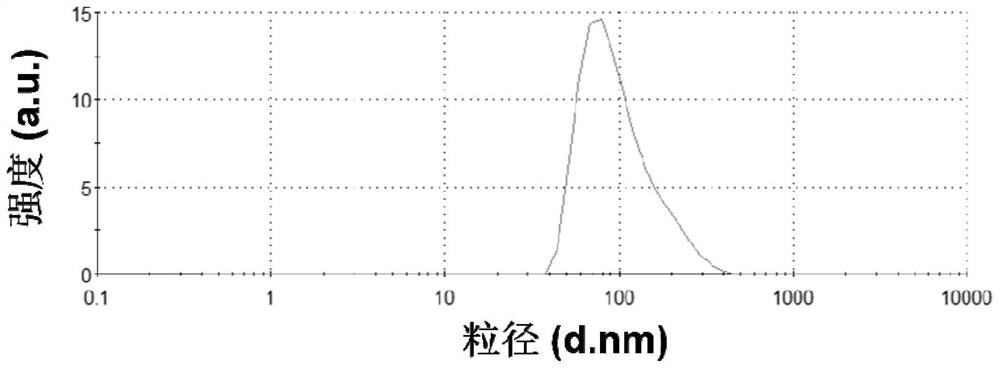
[0065] The nano-medicine provided in this embodiment includes tripterine, the particle size of the nano-medicine is 90nm, and the hydrated particle size is 120nm.
[0066] The preparation method is as follows:
[0067] (1) 5 mg tripterine was dissolved in 1.25 mL dimethyl sulfoxide to obtain a solution A whose molar concentration of tripterine was 10 mM / L;
[0068] (2) 7.8 mg of hyaluronic acid was dissolved in 1 mL of water to obtain a solution B with a hyaluronic acid molar concentration of 10 mM / L;
[0069] (3) 100 μL of the solution A obtained in step (1) was injected into 800 μL of water for three times, injected into deionized water, and magnetically stirred at a speed of 100 rpm at 20° C. to obtain solution C;
[0070] (4) 100 μL of solution B obtained in step (2) was injected into solution C obtained in step (3) and mixed to obtain solution D, which was magnetically stirred at 100 rpm at 20° C. for 24 hours to obtain nanomedicine.
[0071] The nano-medicine provided ...
Embodiment 2
[0073] The nano-medicine provided in this embodiment includes tripterine, the particle size of the nano-medicine is 80nm, and the hydrated particle size is 114.3nm.
[0074] The preparation method is as follows:
[0075] (1) 50mg tripterine was dissolved in 1.25mL methanol to obtain solution A;
[0076] (2) 78 mg of hyaluronic acid was dissolved in 1 mL of water to obtain solution B;
[0077] (3) 10 μL of the solution A obtained in step (1) was injected into 890 μL of water for three times, injected into deionized water, and magnetically stirred at 2000 rpm at 25° C. to obtain solution C;
[0078] (4) 100 μL of solution B obtained in step (2) was injected into solution C obtained in step (3) and mixed to obtain solution D, which was magnetically stirred at 25° C. at a speed of 2000 rpm for 12 hours to obtain nanomedicine.
[0079] Carry out dynamic light scattering test (DLS) to the nano-medicine provided in this embodiment, what record is the hydrated particle size of nano-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com