Novel preparation method of tofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the field of medicine, can solve the problems of low yield, high cost, and many impurities in by-products, and achieve the effect of good fluidity and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
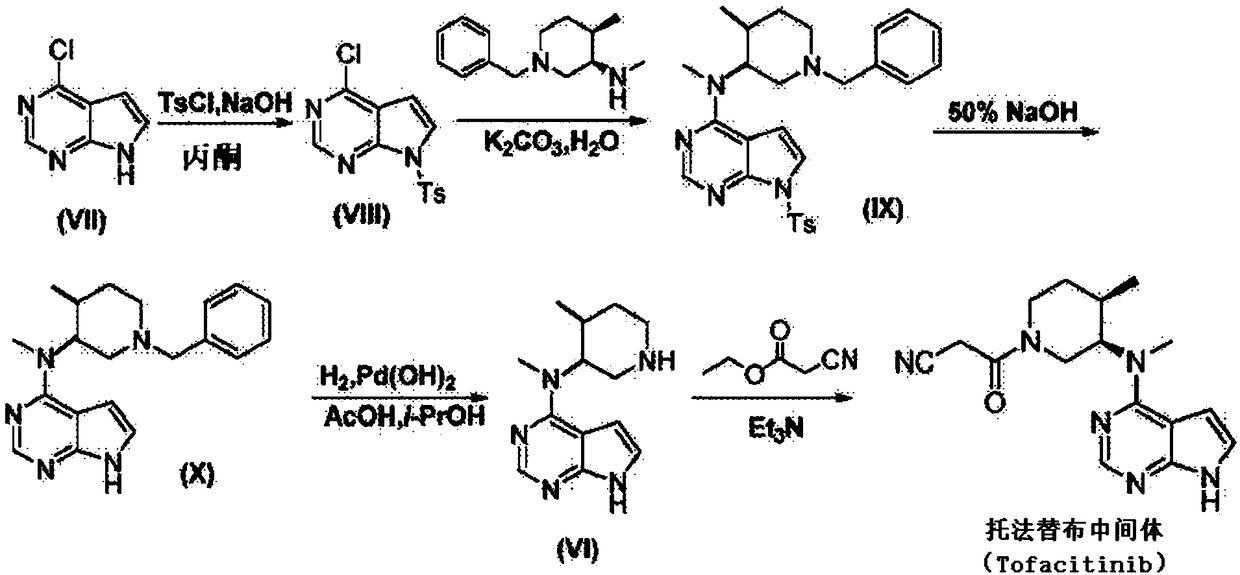
[0035] The invention provides a kind of preparation method of tofacitinib citrate, described method comprises steps:
[0036] (1) Add the crude product of tofacitinib intermediate into the solvent and dissolve it completely at a higher temperature (such as about 65°C), add medicinal activated carbon and activated alumina and stir for adsorption for 0-10min, then cool down to a lower temperature (such as about 30°C) ~40°C), stir and adsorb at a lower temperature for 20-50 minutes, then raise the temperature to a higher temperature (such as about 65°C), and continue to stir and adsorb for 10-40 minutes;
[0037] (2) Remove medicinal activated carbon and activated alumina by filtration, add pre-cooled alkaline aqueous solution to the filtrate, cool to 0-5°C to crystallize, wash and dry to obtain the refined product of tofacitinib intermediate;
[0038] (3) Citric acid monohydrate (C 6 h 8 o 7 ·H 2 O) Dissolve in ethanol aqueous solution, then add the refined tofacitinib inter...
Embodiment 1
[0045] Add 2.0kg of the crude product of tofacitinib intermediate (HPLC purity is about 92%) into 20L ethanol, heat to 65°C and dissolve completely, then add 200g of medicinal activated carbon and 100g of activated alumina and stir for 10min, then continue to stir and cool down to 10 ~15°C, maintain for 30min, then raise the temperature to 65°C, continue stirring for 30min, then filter while hot. Add 50L of sodium bicarbonate solution (mass fraction 0.5%) to the filtrate, cool to 0-5°C, filter after crystallization for 6 hours, wash with cold water and dry to obtain the refined product of tofacitinib intermediate, with a yield of 89.4%, HPLC purity is 99.61%.
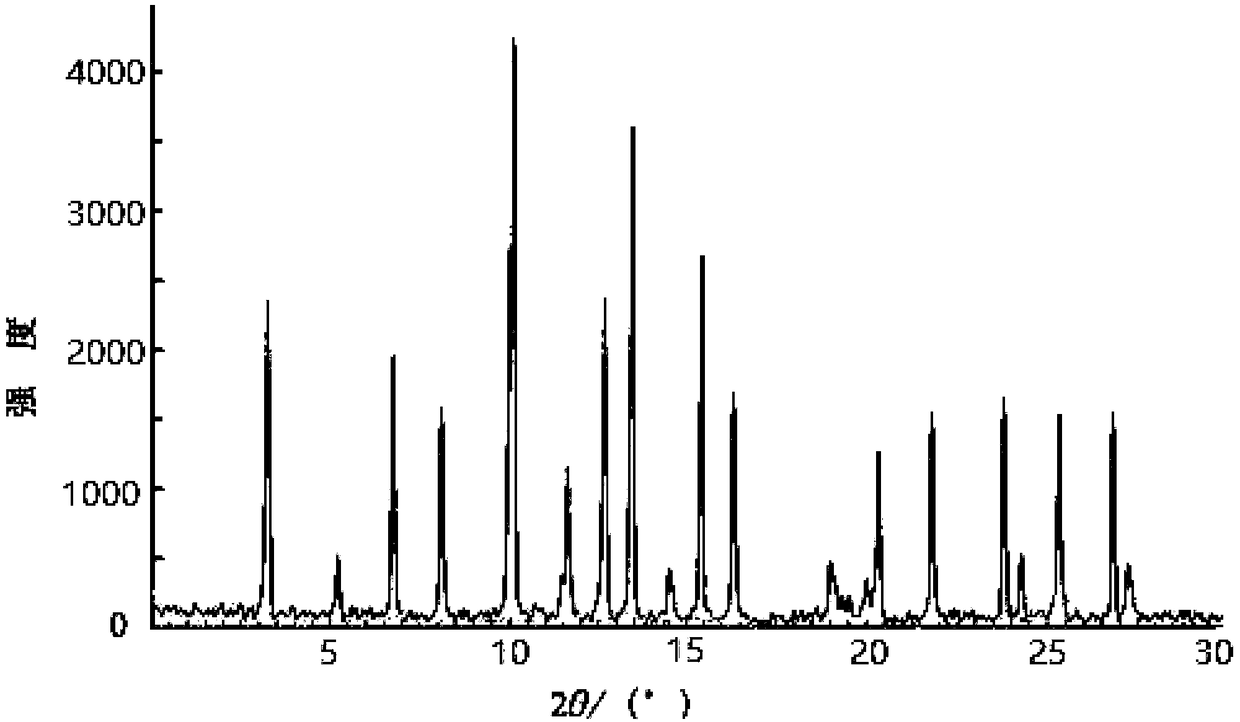
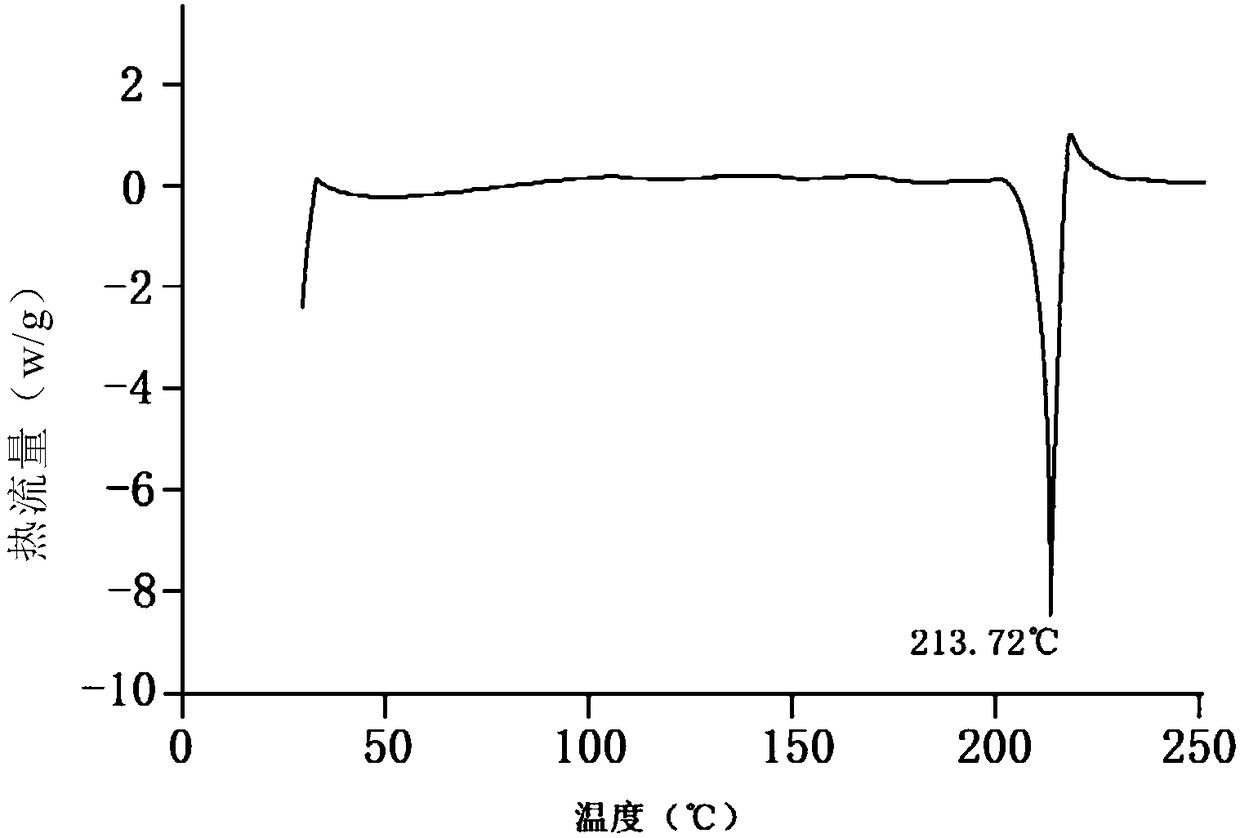
[0046] Citric acid monohydrate (C 6 h 8 o 7 ·H 2 O) 7.4g is dissolved in 150ml ethanol aqueous solution (the volume ratio of ethanol and water is 3:2), then add 10g of tofacitinib intermediate refined product, heat and reflux until completely dissolved, cool to 35°C, and transfer the solution to ultrasonic In the p...
Embodiment 2
[0052] Add 1.0kg of the crude product of tofacitinib intermediate (HPLC purity is about 92%) into 15L ethanol, heat to 65°C and dissolve completely, then add 150g of medicinal activated carbon and 100g of activated alumina and stir for 10min, then continue to stir and cool down to 10 ~15°C, maintain for 30min, then raise the temperature to 65°C, continue stirring for 35min, then filter while hot. Add 40L of sodium bicarbonate solution (mass fraction 0.5%) to the filtrate, cool to 0-5°C, filter after crystallization for 6 hours, wash with cold water and dry to obtain the refined product of tofacitinib intermediate, with a yield of 86.3%, HPLC purity is 99.81%.
[0053] Citric acid monohydrate (C 6 h 8 o 7 ·H 2 O) 7.4g was dissolved in 200ml ethanol aqueous solution (the volume ratio of ethanol and water was 3:2), then added 10g of tofacitinib intermediate refined product, heated to reflux until completely dissolved, cooled to 35°C, and the solution was transferred to ultras...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com