Hydroxyalkyl starch conjugate, and preparation method and application thereof
A technology of hydroxyalkyl starch and hydroxyethyl starch, which is applied in the directions of pharmaceutical combinations, pharmaceutical formulations, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
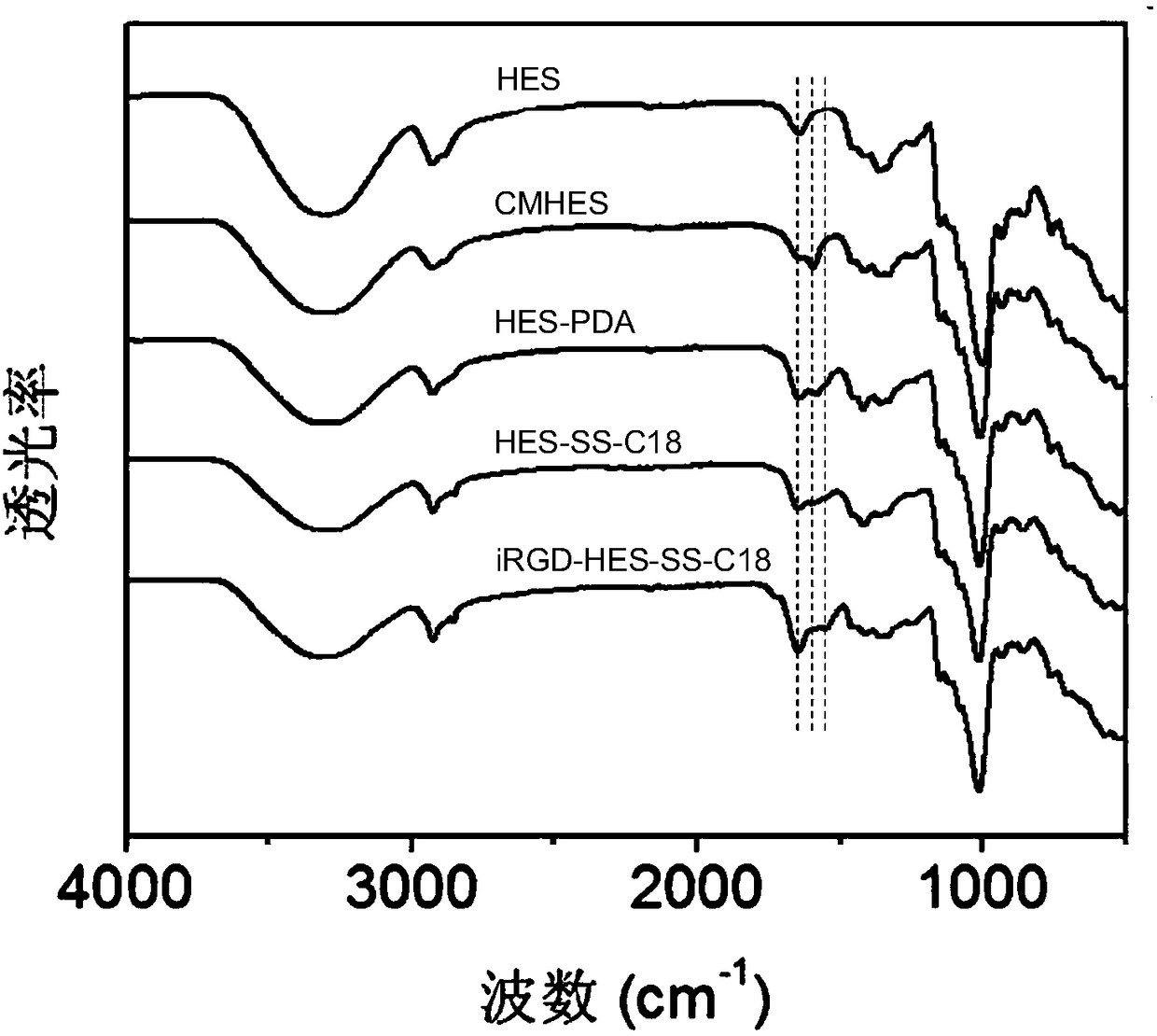
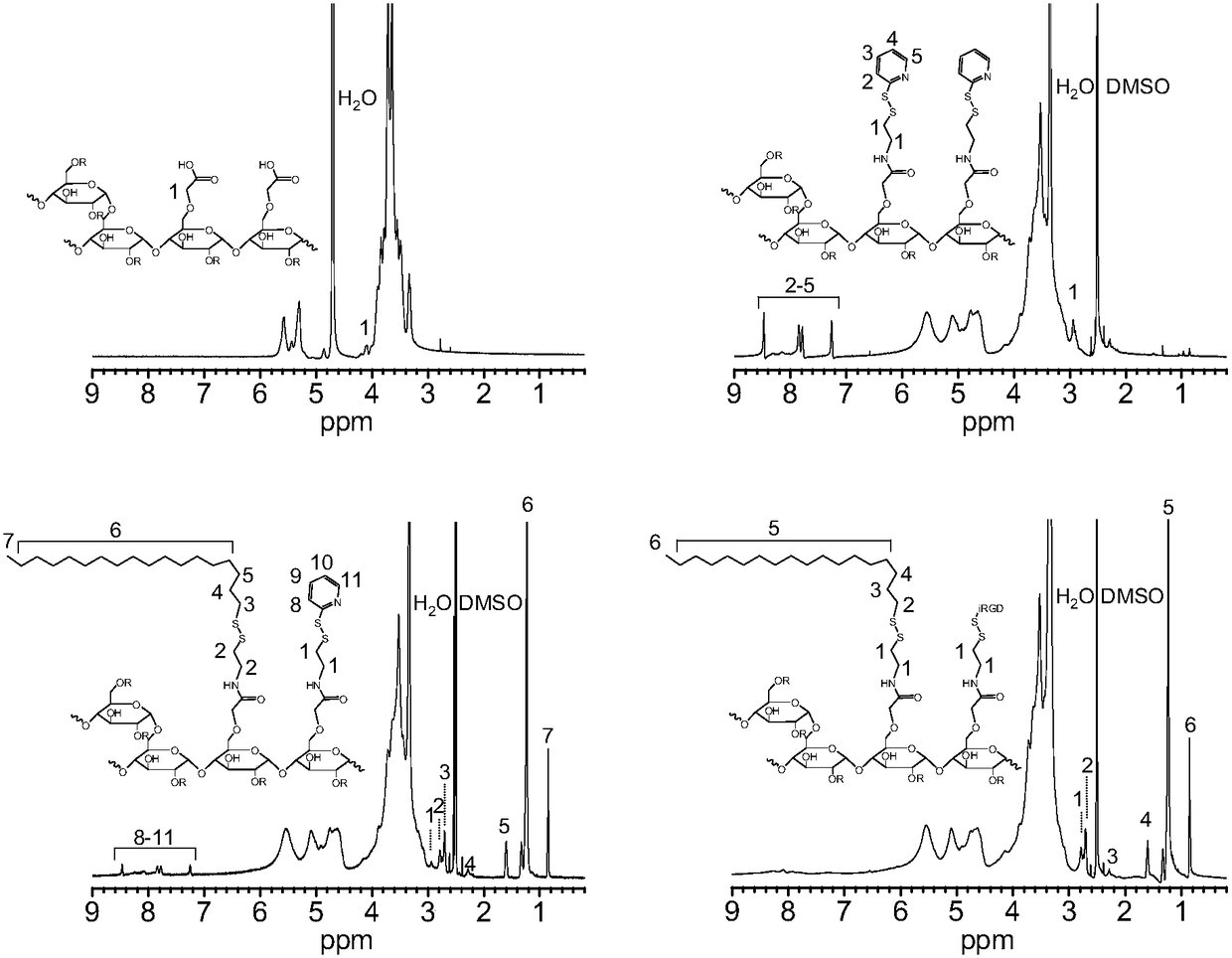
Image
Examples
Embodiment 1
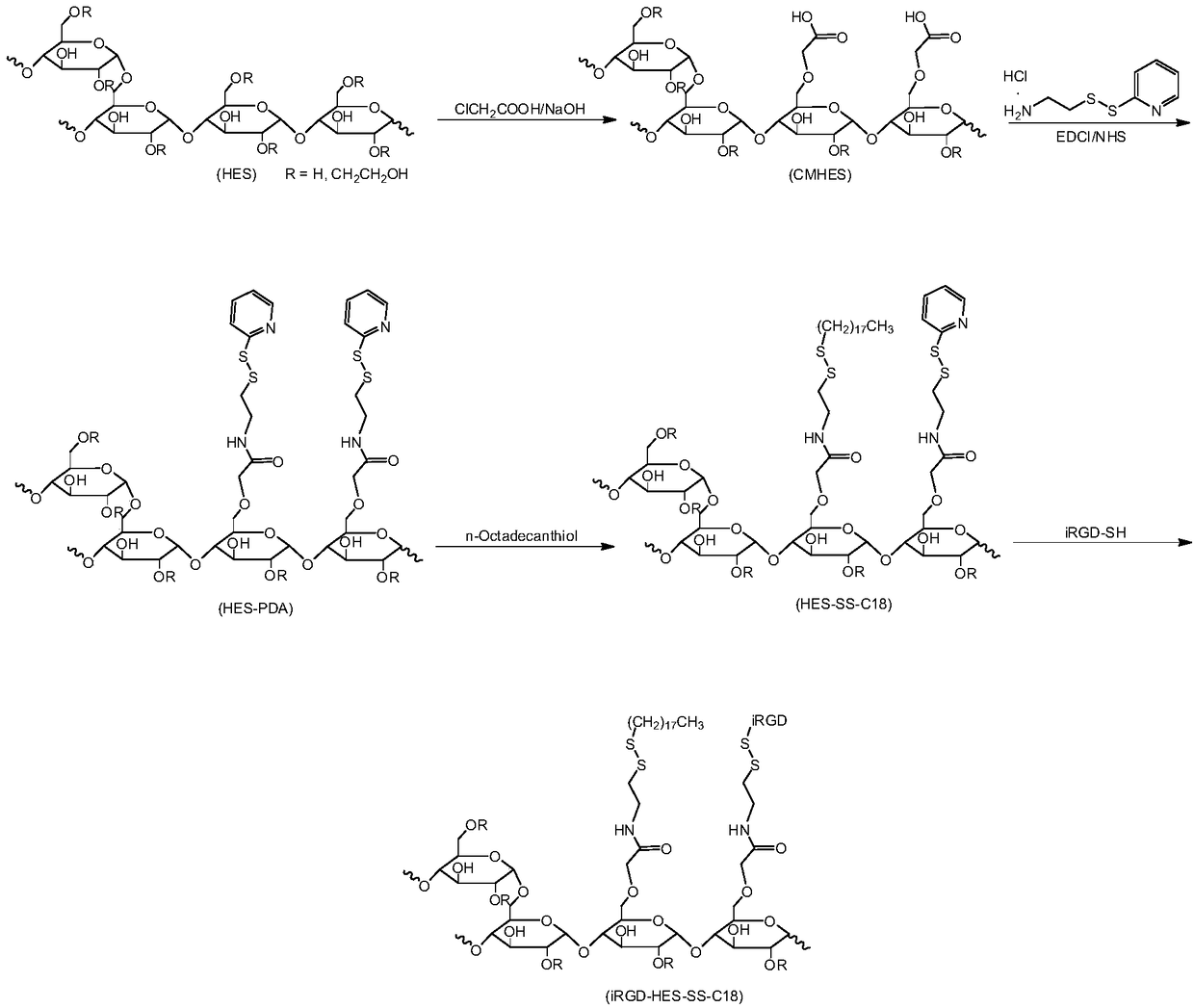
[0081] The present embodiment provides a hydroxyethyl starch conjugate, comprising the steps of:
[0082] (1) Dissolve 1.00 g of hydroxyethyl starch (sugar ring is 5.6 mmol) in 20 mL of ultrapure water, add 0.85 g (21.2 mmol) of sodium hydroxide to activate the hydroxyl group, and stir at room temperature for 0.5 h to obtain the reaction solution A, and then to the reaction Add 1.00 g (10.6 mmol) of chloroacetic acid to solution A, stir at room temperature to dissolve, then move to 70°C oil bath and stir for 3 hours to obtain reaction solution B;
[0083] (2) Pour the reaction solution B obtained in step (1) into 200 mL of methanol and stir to obtain a suspension C;
[0084] (3) Filtrate the suspension C obtained in step (2) to obtain a white precipitate, wash the precipitate three times with methanol, 50 mL each time, and then vacuum-dry at room temperature to obtain a dry white precipitate;
[0085] (4) Redissolve the dry white precipitate obtained in step (3) in ultrapure ...
Embodiment 2
[0095] The present embodiment provides a hydroxyethyl starch conjugate, comprising the steps of:
[0096] Steps (1)~(6) are identical with embodiment 1
[0097] (7) 195.6 mg (2-pyridyl is 0.11 mmol) of the hydroxyethyl starch-2-(2-pyridyldithio)ethylamine conjugate obtained in step (6) was dissolved in 5 mL dimethyl sulfoxide, Add 17.2mg (0.06mmol) of n-octadecylmercaptan, and stir at room temperature for 24h to obtain reaction solution E;
[0098] (8) Pour the reaction solution E obtained in step (7) into 50 mL of ethanol / ether mixed solvent (1:1, V / V), and stir to obtain a suspension F;
[0099] (9) Filter the suspension F obtained in step (8) to obtain a white precipitate, wash the white precipitate three times with ethanol / ether mixed solvent (1:1, V / V), 10 mL each time, and then vacuum-dry at room temperature , to obtain a dry white precipitate;
[0100] (10) Disperse the dry white precipitate obtained in step (9) in ultrapure water, and dialyze with ultrapure water fo...
Embodiment 3
[0104] The present embodiment provides a hydroxyethyl starch conjugate, comprising the steps of:
[0105] Steps (1)~(6) are identical with embodiment 1
[0106] (7) 195.6 mg (2-pyridyl is 0.11 mmol) of the hydroxyethyl starch-2-(2-pyridyldithio)ethylamine conjugate obtained in step (6) was dissolved in 5 mL dimethyl sulfoxide, Add 22.9mg (0.08mmol) of n-octadecylmercaptan, stir and react at room temperature for 24h to obtain reaction solution E;
[0107] (8) Pour the reaction solution E obtained in step (7) into 50 mL of ethanol / ether mixed solvent (1:1, V / V), and stir to obtain a suspension F;
[0108] (9) Filter the suspension F obtained in step (8) to obtain a white precipitate, wash the white precipitate three times with ethanol / ether mixed solvent (1:1, V / V), 10 mL each time, and then vacuum-dry at room temperature , to obtain a dry white precipitate;
[0109] (10) Disperse the dry white precipitate obtained in step (9) in ultrapure water, and dialyze with ultrapure wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com