Construction method and application of Escherichia coli cold shock assistant dissolving type expression plasmids
A technology of Escherichia coli and expression plasmids, which is applied in the field of bioengineering to achieve good solubility, improved solubility, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Construction of Escherichia coli Cold Shock Solubilizing Expression Vector
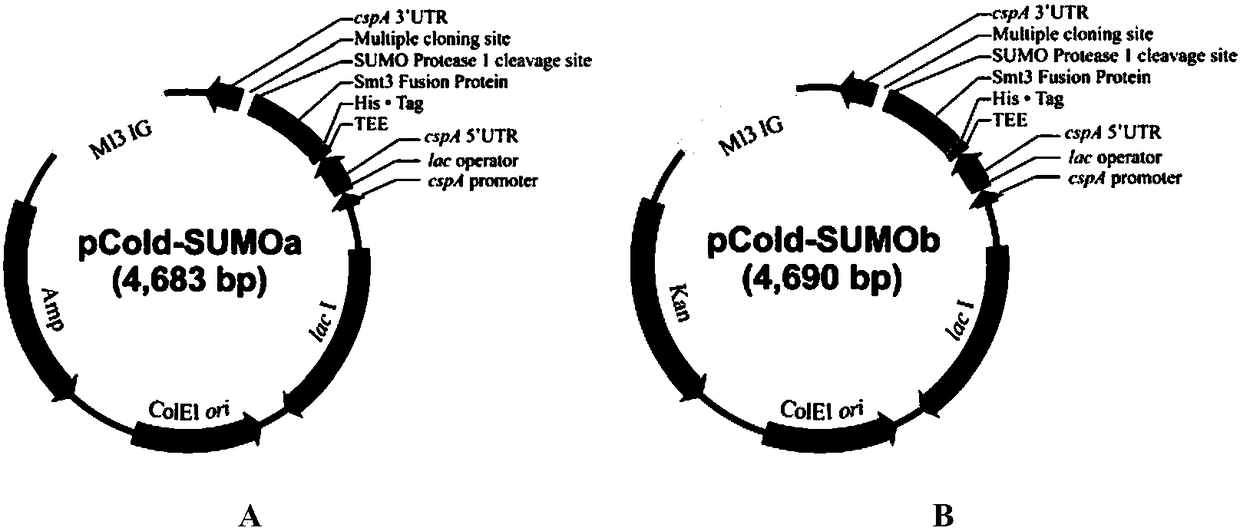
[0044] 1. Construction of ampicillin-resistant pCold-SUMOa plasmid
[0045] With the pE-SUMOpro Kan plasmid of LifeSensors company as a template, primers SUMO-F (sequence shown in SEQ ID No.3) and SUMO-R (sequence shown in SEQ ID No.4) were used to amplify the Smt3 fusion protein sequence ( The sequence is shown in SEQ ID NO.19). At the same time, using the pCold TF plasmid digested by Sal I as a template, the primers pCold-F (sequence shown in SEQ ID No.1) and pCold-R (sequence shown in SEQ ID No.2) were amplified to obtain pCold plasmid backbone fragment. Then adopt the seamless cloning technique to connect the above two fragments to obtain a recombinant plasmid, which is called pCold-SUMOa (such as figure 1 shown). Since the Smt3 fusion protein gene sequence contains two restriction sites, EcoR I and Pst I, the pCold TF plasmid after single digestion with SalI was selected as ...
Embodiment 2
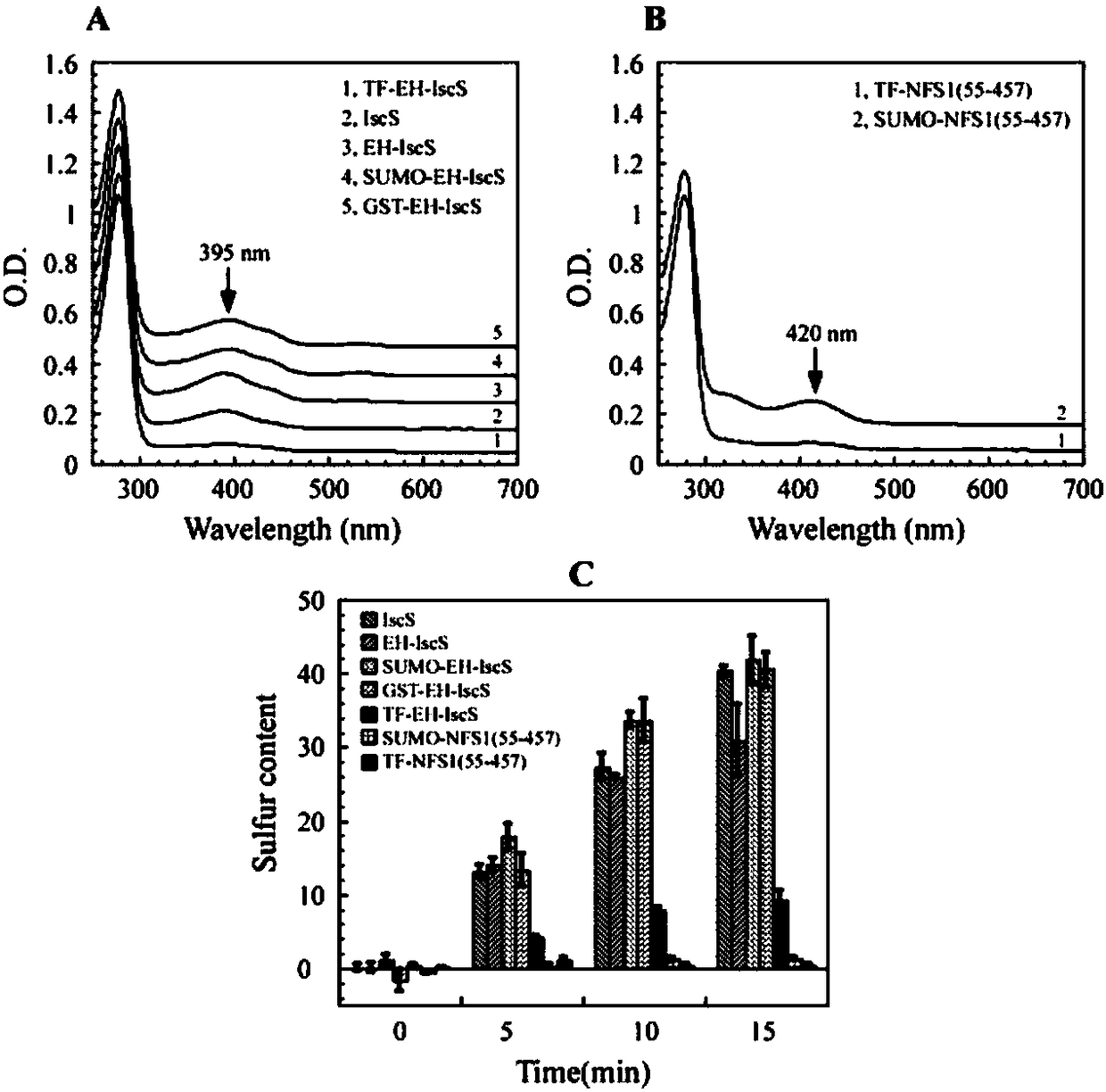
[0050] Example 2. Construction of recombinant plasmid expressing cysteine desulfurase
[0051] 1. Construction of a recombinant plasmid expressing Escherichia coli cysteine desulfurase
[0052] Using the Escherichia coli E.coli MC4100 genome as a template, the primers IscS-pCold-F (sequence shown in SEQ ID No.9) and IscS-pCold-R (sequence shown in SEQ ID No.10) were amplified to obtain Escherichia coli IscS coding box gene. The pCold I plasmid was digested with Kpn I, and the digested vector fragment was recovered by gel. Then, the seamless cloning kit was used to construct the recombinant plasmid IscS-pCold I, and the recombinant plasmid was sent to BGI for sequencing identification.
[0053] 2. Construction of recombinant plasmid expressing human cysteine desulfurase
[0054] With the NFS1(55-457)-pET28b plasmid as a template, primers m-NFS1-pCold-F (sequence shown in SEQ ID NO.11) / SUMO-m-NFS1-F (sequence shown in SEQ ID NO.13) were used ) and m-NFS1-pCold-R (seque...
Embodiment 3
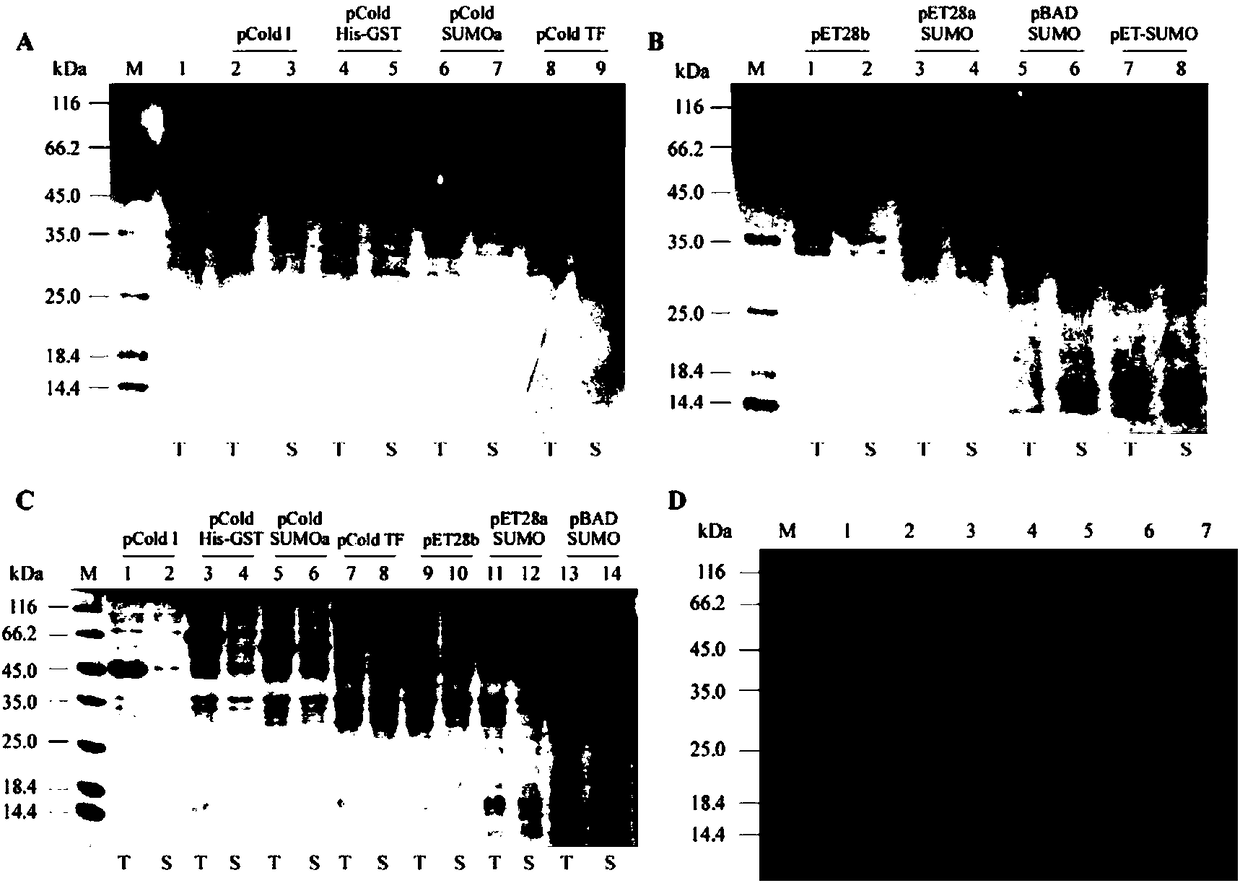
[0111] Example 3. Induction and expression condition optimization of cysteine desulfurase
[0112] Pass the recombinant plasmid constructed above through cold CaCl 2 Transformed into BL21(DE3) competent cells. The induction and expression of the recombinant cysteine desulfurase of the present invention, unless otherwise stated, are all operated according to the following experimental steps:
[0113] (1) Inoculate the screened transformant overnight bacteria into LB liquid medium containing 100 μg / ml Amp at a volume ratio of 1:50, and culture with shaking at 37°C and 250rpm;
[0114] (2) When the OD600 value of the bacterial solution is about 0.4-0.5, immediately place the bacterial solution in an ice-water bath for 5 minutes, then move it to 15°C and let it stand for 30 minutes;
[0115] (3) Add IPTG to a final concentration of 100 μM / L, shake and culture at 15° C. and 250 rpm for 24 hours.
[0116] (4) After the culture is completed, use SDS-PAGE to analyze the presenc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com