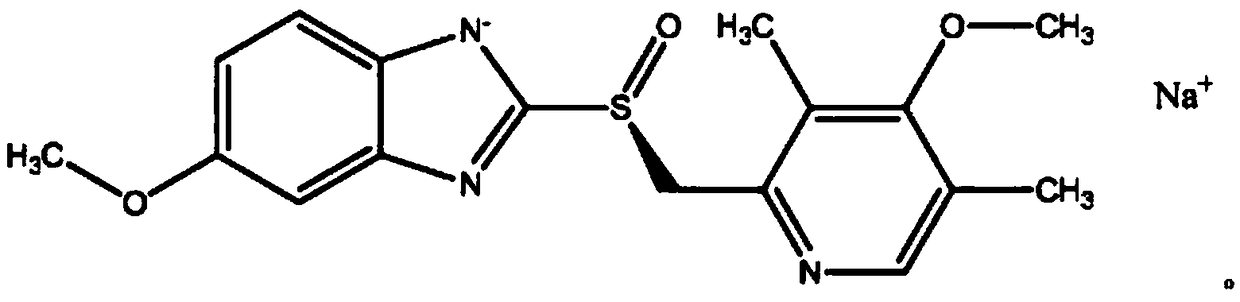
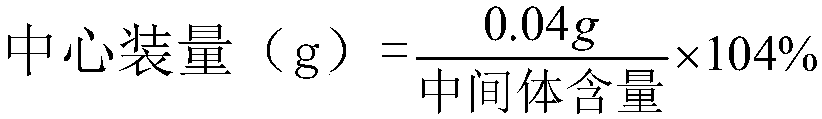Sodium esomeprazole for injection
A technology for esomeprazole sodium and injection, which is applied to medical preparations without active ingredients, medical preparations containing active ingredients, and the digestive system, etc. Calcium drop and other problems, to avoid contact between rubber stopper particles and drugs, avoid abnormal phenomena, and reduce total impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The implementation of the present invention will be described in detail below in conjunction with the examples, but they do not constitute a limitation of the present invention, and are only examples. At the same time, the advantages of the present invention are clearer and easier to understand through the description.
[0047] Esomeprazole sodium for injection is characterized in that: it is esomeprazole sodium for injection with 40 mg specification, and the formula components are:
[0048]
[0049] Sodium hydroxide is a pH regulator; medicinal charcoal and water for injection are substances used in the preparation and finally removed;
[0050] The method for preparing described esomeprazole sodium for injection is characterized in that, comprises the steps:
[0051] Step 1: Pretreatment of bottles, stoppers and aluminum caps
[0052] ① Freeze-dried vials: medium borosilicate vials are cleaned, dried and sterilized, and insoluble particles and visible foreign matt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com