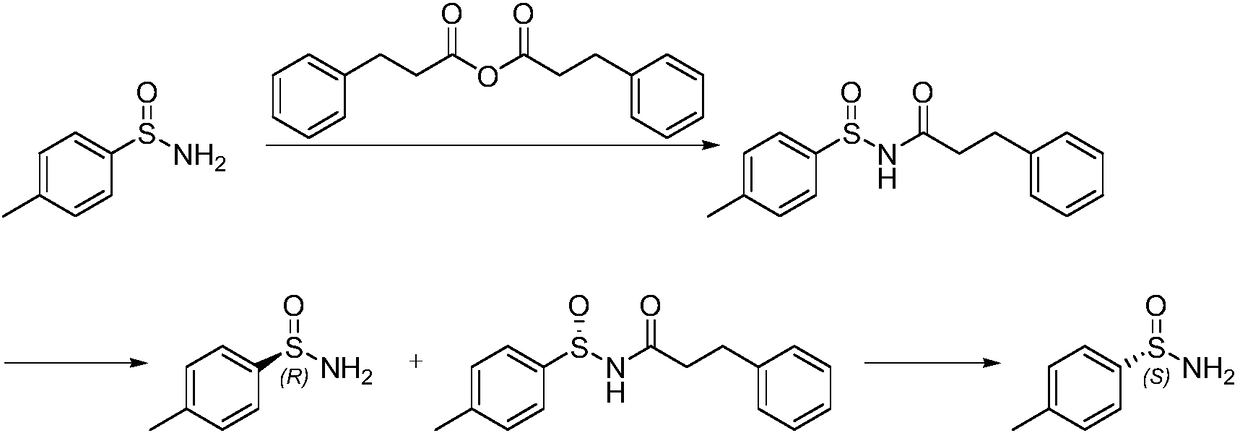
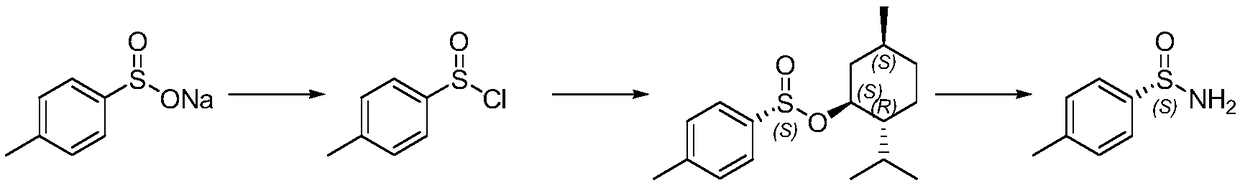
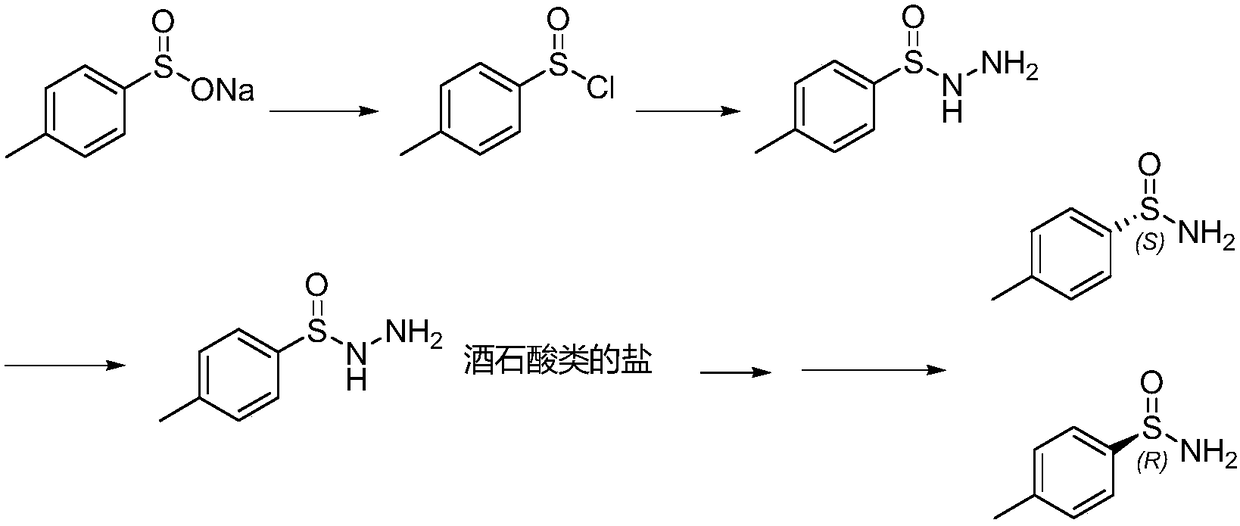
Preparation method of chiral optical pure p-toluene sulfamide
A technology of toluene sulfinamide and sodium toluene sulfinamide is applied in the field of preparation of chiral optically pure p-toluene sulfinamide, which can solve the problems of unfavorable large-scale production, large solvent ratio and high price of LiHMDS, and achieves atomic utilization rate High, improve alkalinity, cost saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The first step: the synthesis of p-toluenesulfinyl chloride.
[0042] (1) Put 267g of toluene, 53.4g of sodium p-toluene sulfinate and 0.1g of N,N-dimethylformamide into a dry and clean 500mL four-necked bottle with an addition funnel, add 57.5g of thionyl chloride dropwise at room temperature, React at 55-65°C for 4 hours, take samples for GC or HPLC detection after derivatization, the raw material is less than 0.3%, concentrate under reduced pressure, replace once with 150g of toluene, then add 50g of toluene to dilute for later use. The external standard yield is 94%.
[0043] (2) Put 267g of benzene, 53.4g of sodium p-toluenesulfinate and 0.1g of N,N-dimethylformamide into a dry and clean 500mL four-necked bottle with an addition funnel, add 57.5g of thionyl chloride dropwise at room temperature, and Reaction at 55-65°C for 4 hours, sampled by GC or HPLC after derivatization, the raw material is less than 0.3%, concentrated under reduced pressure, replaced once wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com