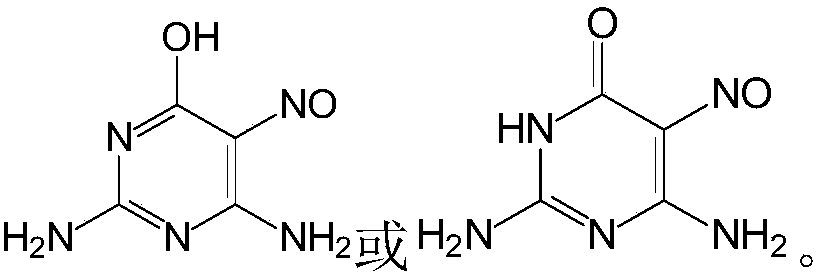
Preparation methods of 2,4-diamido-5-nitroso-6-hydroxypyridine and guanine
A technology of hydroxypyrimidine and nitroso, which is applied in the field of preparation of 2,4-diamino-5-nitroso-6-hydroxypyrimidine and guanine, which can solve the problem that dilute formic acid solution cannot be recycled and applied mechanically, which increases the cost of preparation , Increase the cost of guanine preparation, etc., to achieve the effect of being suitable for large-scale production, reducing biodegradability, and facilitating recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
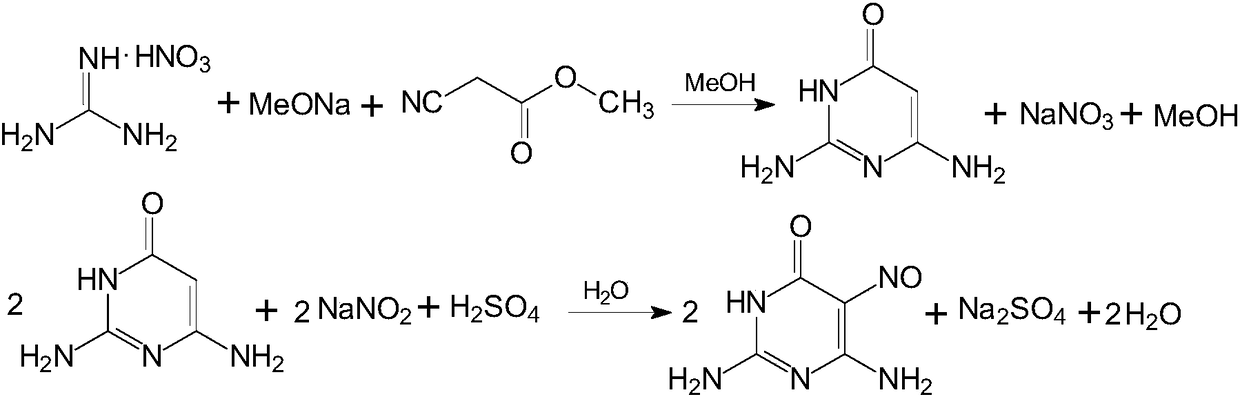
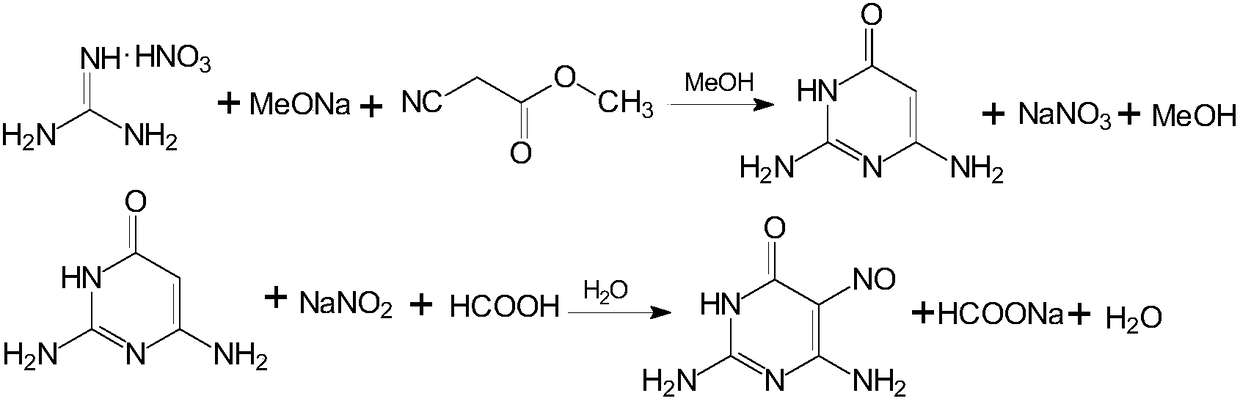
[0054] Put 950Kg of sodium methoxide methanol solution into a 3KL enamel reaction kettle, start stirring under nitrogen protection, and then put in 325Kg of guanidine nitrate. After feeding, the temperature was raised to reflux for 90 minutes.
[0055] Then, dropwise addition of 253 Kg of methyl cyanoacetate was started. After dripping, continue the reflux ring closure reaction for 4 hours to end the reaction.
[0056] After the reaction is over, transfer the material to a 5KL enamel salt crystallization kettle, add 2000L of anhydrous methanol recovered by distillation in the previous batch, and continue the reflux reaction for 30 minutes. Then, lower the temperature to 50°C, and keep stirring for 60 minutes. Pressure filter with nitrogen to dryness, then wash the filter cake with 150L of anhydrous methanol recovered from the previous batch of distillation, combine the filtrate and washing liquid, and transfer it to a 5KL enamel concentration kettle; the filter cake is the b...
Embodiment 2
[0063] Put 950Kg of sodium methoxide methanol solution into a 3KL enamel reaction kettle, start stirring under nitrogen protection, and then put in 325Kg of guanidine nitrate. After feeding, the temperature was raised to reflux for 30 minutes.
[0064] Then, dropwise addition of 253 Kg of methyl cyanoacetate was started. After dropping, continue the reflux ring closure reaction for 2 hours to end the reaction.
[0065] After the reaction is over, transfer the material to a 5KL enamel salt crystallization kettle, add 2000L of anhydrous methanol recovered by distillation in the previous batch, and continue the reflux reaction for 60 minutes. Then, the temperature was lowered to 60°C, and the mixture was kept stirring for 30 minutes. Pressure filter with nitrogen until dry, then wash the filter cake with 150Kg of anhydrous methanol recovered from the previous batch of distillation, combine the filtrate and washing liquid, and transfer it to a 5KL enamel concentration kettle; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com