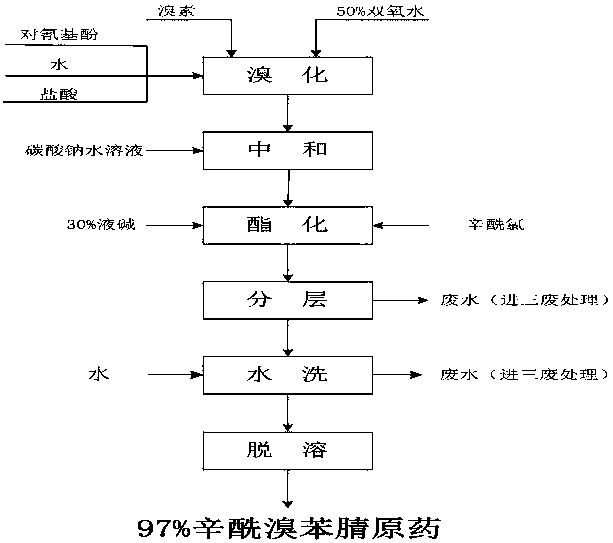
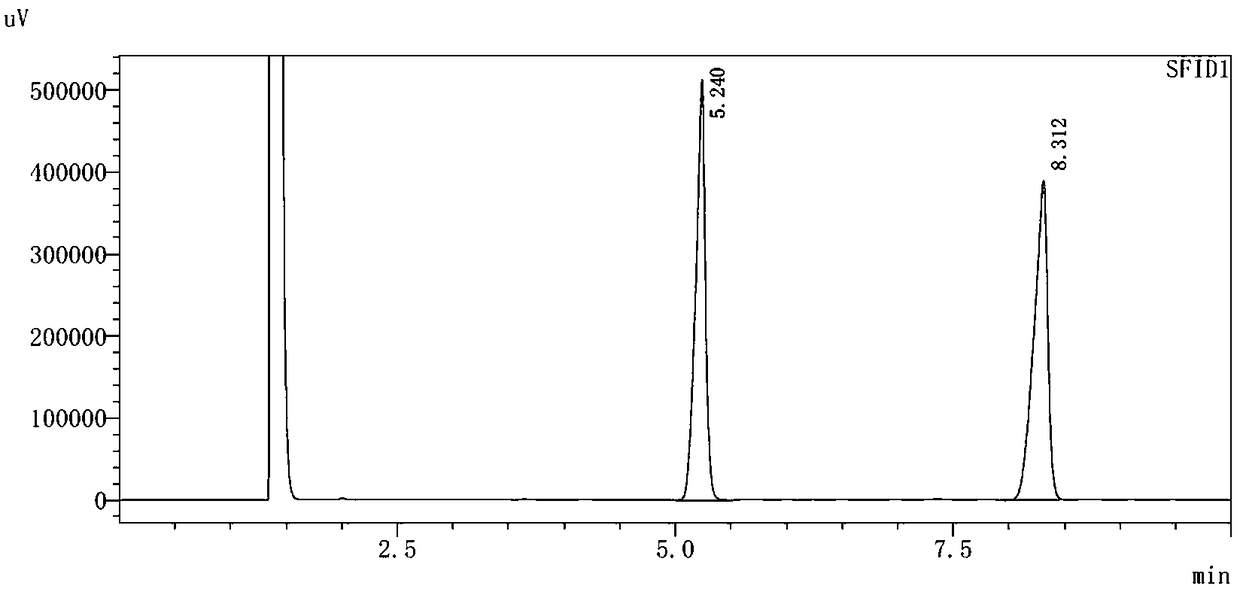
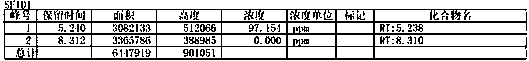Preparation method of high-purity bromoxynil octanoate
A technology of pure octanoyl bromoxynil and bromination reaction, applied in the field of preparation of high-purity octanoyl bromoxynil, can solve the problems of high transport requirement, high cost, large steric resistance of octanoyl ester group, etc., and achieves reduction of recycling effect of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 500ml four-neck flask, add 23.8g (0.2mol) of p-hydroxybenzonitrile and 200g of water, raise the temperature to 50±5°C, and start to drop 32g (0.2mol) of bromine and 27.2g (0.4mol) of 50% hydrogen peroxide, The dropwise addition time is about 4 hours. After the dropwise addition, keep warm for 30 minutes, take a sample test, and after the reaction is qualified, add a small amount of sodium carbonate to destroy the hydrogen peroxide, and use the starch potassium iodide test paper to pass the test. Then cool down to 25±5°C and add 100g of toluene. Add 26g (0.2mol) of 30% sodium hydroxide solution and 33.2g (0.204mol) octanoyl chloride dropwise, add dropwise for 2 hours, keep warm for 0.5 hour, take samples, and after the reaction is qualified, static layering. Then add 100m1 process water, stir for 10 minutes, pour into a separatory funnel, separate the water phase, and carry out negative pressure precipitation for the organic phase, sampling and testing, the content i...
Embodiment 2
[0020] In a 500ml four-necked flask, add 23.8g (0.2mol) of p-hydroxybenzonitrile and 200g of water, raise the temperature to 60±5°C, and start to drop 32g (0.2mol) of bromine and 27.88g (0.41mol) of 50% hydrogen peroxide, The dropwise addition time is about 4 hours. After the dropwise addition, keep warm for 30 minutes, and take samples for testing. After the reaction is qualified, add a small amount of sodium carbonate to destroy the hydrogen peroxide, and use starch potassium iodide test paper to test it. Then cool down to 5±5°C and add 100g of toluene. Add 29.33g (0.22mol) of 30% sodium hydroxide solution and 33.2g (0.204mol) of octanoyl chloride dropwise, add dropwise for 2 hours, keep warm for 0.5 hours, take samples, and after the reaction is qualified, static layering. Then add 100m1 process water, stir for 10 minutes, pour into a separatory funnel, separate the water phase, and carry out negative pressure precipitation for the organic phase, sampling and testing, the co...
Embodiment 3
[0022] In a 500ml four-necked flask, add 23.8g (0.2mol) of p-hydroxybenzonitrile and 200g of water, raise the temperature to 60±5°C, and start to drop 32g (0.2mol) of bromine and 27.88g (0.41mol) of 50% hydrogen peroxide, The dropwise addition time is about 4 hours. After the dropwise addition is completed, keep warm for 30 minutes, and take a sample test. After the reaction is qualified, add a small amount of sodium carbonate to destroy the hydrogen peroxide. Use starch potassium iodide test paper to test the pass, then cool down to 5±5°C and add dichloroethane 100g, add 29.33g (0.22mol) of 30% sodium hydroxide solution and 33.2g (0.204mol) octanoyl chloride dropwise at the same time, dropwise for 2 hours, keep warm for 0.5 hours, take samples, after the reaction is qualified, static layering. Then add 100m1 process water, stir for 10 minutes, pour into the separatory funnel, separate the water phase, and carry out negative pressure precipitation for the organic phase, samplin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com