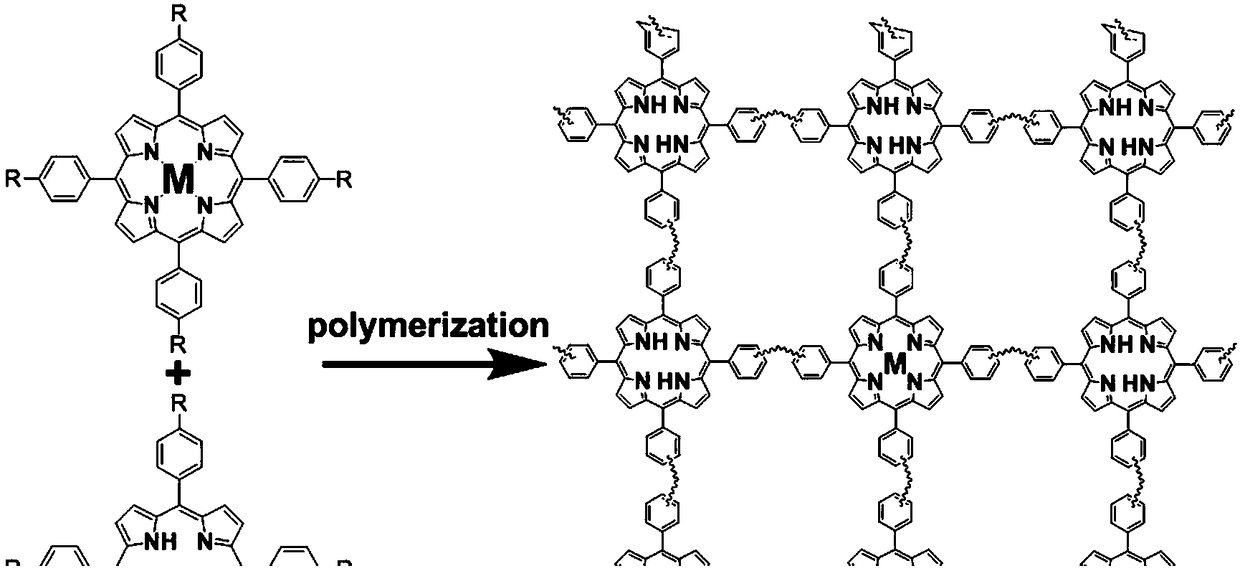
Preparation method of nitrogen-doped porous carbon-supported metal monoatomic material
A nitrogen-doped porous carbon and metal-loaded technology, which is applied in the field of materials science and engineering, can solve the problems of low metal content, poor adjustability, and complicated operation, and achieve rich research, wide application, and meet the requirements of experimental diversification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
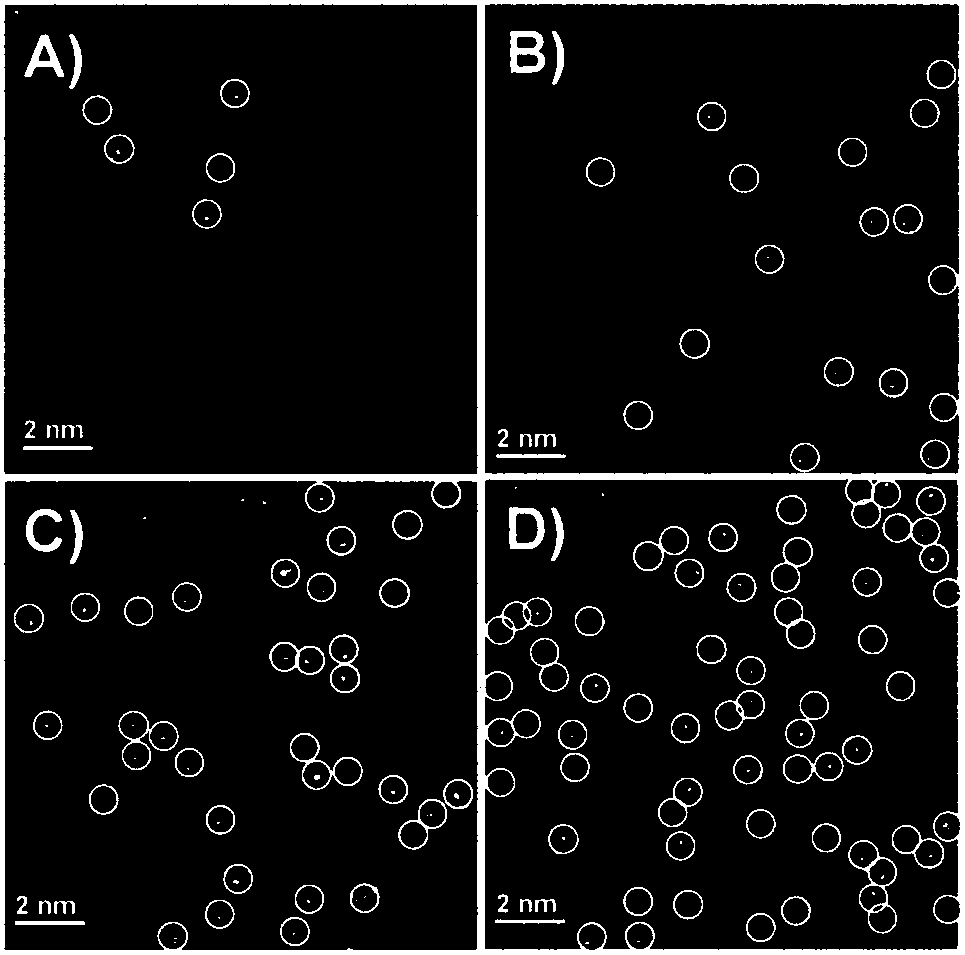
[0037] Add TPP in 100 ml polytetrafluoroethylene lining: PtTPP = 0.922 g : 0.008 g (99 wt% : 1wt%), 30 ml dichloromethane, 3.195 g anhydrous aluminum chloride; Magnetic stirring in a stainless steel reactor, reaction The temperature was 60 °C, and the reaction time was 48 h; after the Friedel-Crafts alkylation reaction, the Soxhlet extraction and purification were performed with THF, acetone, and methanol, and vacuum 80 o C dried overnight. The polymer product of the Friedel-Crafts alkylation reaction was carbonized at 600 °C in a tube furnace, the gas flow of carbonization was selected from argon, the carbonization time was 5 h, and Pt was obtained after cooling to room temperature. 1 / N-C metal single atom. ICP quantification: Pt—0.06 wt%. Spherically aberration-corrected TEM such as figure 2 As shown in A.
Embodiment 2
[0039] Add TPP in 100 ml polytetrafluoroethylene liner: PtTPP = 0.922 g : 0.015 g (98 wt% : 2wt%), 30 ml methylene chloride, 3.195 g anhydrous aluminum chloride; magnetic stirring in a stainless steel reactor, reaction The temperature was 60 °C, and the reaction time was 48 h; after the Friedel-Crafts alkylation reaction, the Soxhlet extraction and purification were performed with THF, acetone, and methanol, and vacuum 80 o C dried overnight. The polymer product of the Friedel-Crafts alkylation reaction was carbonized at 600 °C in a tube furnace, the gas flow of carbonization was selected from argon, the carbonization time was 5 h, and Pt was obtained after cooling to room temperature. 1 / N-C metal single atom. ICP quantification: Pt—0.21 wt%. Spherically aberration-corrected TEM such as figure 2 Shown in B.
Embodiment 3
[0041] Add TPP in 100 ml polytetrafluoroethylene lining: PtTPP = 0.922 g : 0.030 g (97 wt% : 3wt%), 30 ml dichloromethane, 3.195 g anhydrous aluminum chloride; Magnetic stirring in a stainless steel reactor, reaction The temperature was 60 °C, and the reaction time was 48 h; after the Friedel-Crafts alkylation reaction, the Soxhlet extraction and purification were performed with THF, acetone, and methanol, and vacuum 80 o C dried overnight. The polymer product of the Friedel-Crafts alkylation reaction was carbonized at 600 °C in a tube furnace, the gas flow of carbonization was selected from argon, the carbonization time was 5 h, and Pt was obtained after cooling to room temperature. 1 / N-C metal single atom. ICP quantification: Pt—0.58 wt%. Spherically aberration-corrected TEM such as figure 2 C shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com