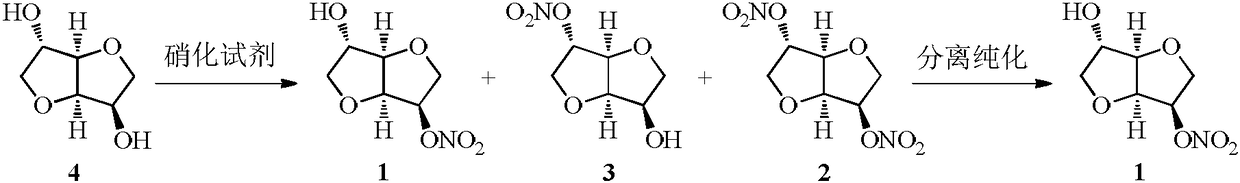
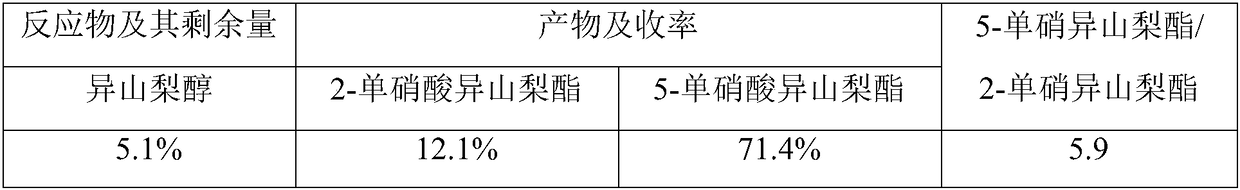
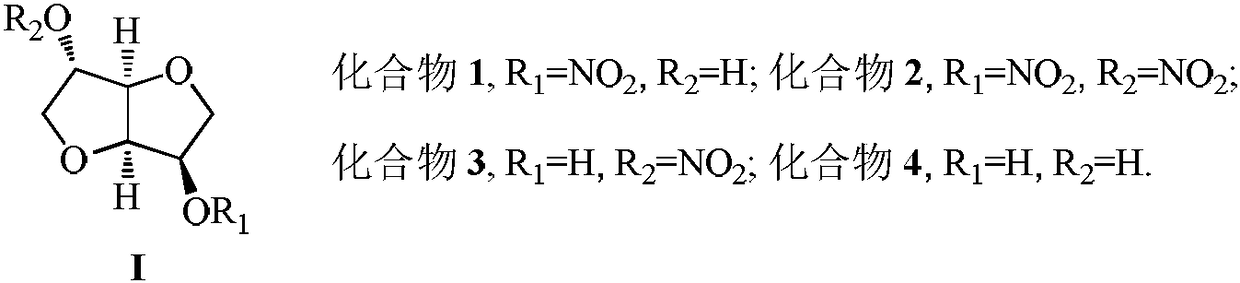Method for synthesizing 5-isosorbide mononitrate through micro-channel reactor
A technology of isosorbide dinitrate and microchannel reactor, which is applied in organic chemistry and other fields, can solve problems such as poor reaction selectivity, low yield, and potential safety hazards in the process, and achieve less side reactions, large specific surface area, and easy reaction conditions The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of nitrating reagent: Add acetic anhydride (260.0g, 2.5mol) into a 500mL dry three-neck flask, control the temperature at 0-10°C, slowly add fuming nitric acid (108.0g, 1.7mol) dropwise, add dropwise When finished, keep warm for later use.
[0047] (2) Preparation of isosorbide solution: in a 2L dry there-necked flask, add isosorbide (250.0g, 1.7mol), isosorbide dinitrate (12.5g, 0.053mol), add 750mL acetic acid / ethyl acetate ( v / v=2:1) mixed solvent, stirred and dissolved for later use.
[0048] (3) Feed nitrating reagent and isosorbide liquid into microchannel reactor by respective metering pumps and carry out mixed reaction, set nitrating reagent flow rate as 15mL / min, the flow rate of isosorbide liquid is 45mL / min, control reaction The temperature is 0°C, 5 templates are connected in series, the liquid holding capacity is 50mL, and the reaction time of the reaction solution in the microchannel reactor is 50s.
[0049] (4) After the reaction efflu...
Embodiment 2
[0054] (1) Preparation of nitrating reagent: In a 1L dry three-necked flask, add acetic anhydride (347.0g, 3.4mol), control the temperature at 0-10°C, slowly add fuming nitric acid (108.0g, 1.7mol) dropwise, add dropwise When finished, keep warm for later use.
[0055] (2) Preparation of isosorbide solution: in a 2L dry there-necked flask, add isosorbide (250.0g, 1.7mol), isosorbide dinitrate (37.5g, 0.159mol), add 750mL acetic acid / dichloromethane ( v / v=1:2) mixed solvent, stirred and dissolved for later use.
[0056] (3) Feed nitrating reagent and isosorbide liquid into the microchannel reactor by respective metering pumps and carry out mixed reaction, setting nitrating reagent flow rate is 5mL / min, and the flow rate of isosorbide liquid is 15mL / min, control reaction The temperature is 20°C, 10 templates are connected in series, the liquid holding capacity is 100mL, and the reaction time of the reaction solution in the microchannel reactor is 300s.
[0057] (4) After the r...
Embodiment 3
[0062] (1) Preparation of nitrating reagent: In a 1L dry three-necked flask, add acetic anhydride (347.0g, 3.4mol), control the temperature at 0-10°C, slowly add fuming nitric acid (108.0g, 1.7mol) dropwise, add dropwise When finished, keep warm for later use.
[0063] (2) Preparation of isosorbide solution: In a 2L dry three-necked flask, add isosorbide (250.0g, 1.7mol), isosorbide dinitrate (75.0g, 0.318mol), add 950mL acetic acid / dichloromethane / A mixed solvent of tetrahydrofuran (v / v / v=3:1:1), stirred and dissolved for later use.
[0064] (3) Feed nitrating reagent and isosorbide liquid into microchannel reactor by respective metering pumps and carry out mixed reaction, set nitrating reagent flow rate as 20mL / min, the flow rate of isosorbide liquid is 50mL / min, control reaction The temperature is 10°C, 5 templates are connected in series, the liquid holding capacity is 50mL, and the reaction time of the reaction solution in the microchannel reactor is 43s.
[0065] (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com