Electrochromic material and preparation method thereof
An electrochromic material and nickel oxide technology, applied in the field of electrochromic materials and their preparation, can solve the problems of single color change, slow electrochromic speed, etc., and achieve a large dimming range, increase contact area, and large activity The effect of reaction area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
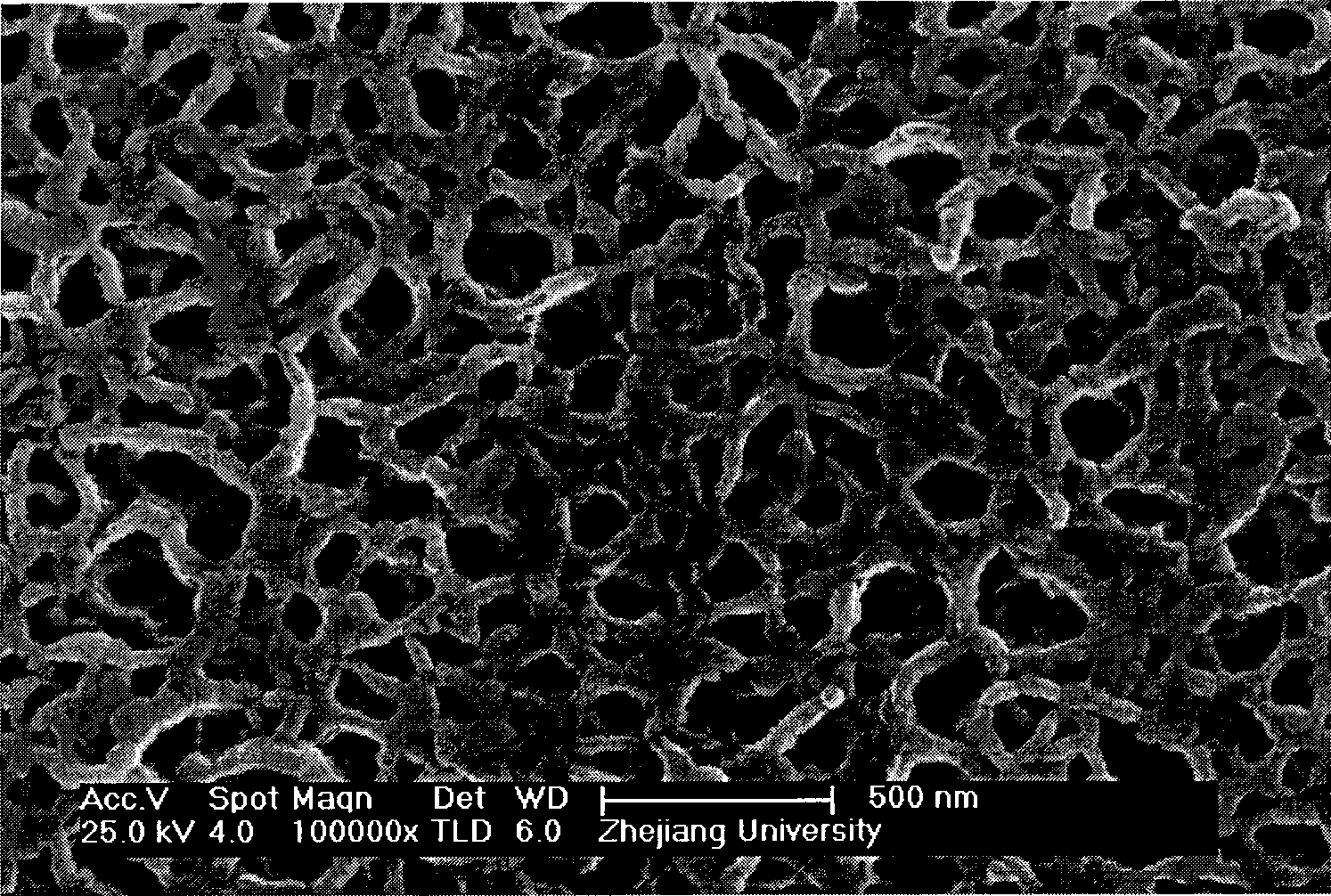
[0016] Weigh 6g of nickel sulfate and 2g of potassium persulfate into a beaker, then add 100mL of deionized water and stir until completely dissolved. Then put the clean ITO glass vertically on the wall of the beaker, its conductive surface faces the solution, and the non-conductive surface is sealed with insulating glue. Then add 6 g of ammonia water with a mass concentration of 20%, stir and react at 35° C. for 10 minutes, take out the sample, dry it naturally, and calcinate it under argon at 350° C. for 1 hour to prepare a porous nickel oxide film.
[0017] Weigh 1.6g of lithium perchlorate and dissolve it in 80g of acetonitrile solution, stir until completely dissolved. Then add 0.16 g of 3,4-ethylenedioxythiophene, stir and dissolve, and pass argon gas through the whole electrolyte for 30 minutes to remove oxygen. Then the electrolyte solution was transferred to an electrochemical three-electrode system, in which the porous nickel oxide film was used as the working elect...
Embodiment 2
[0019] Weigh 24g of nickel sulfate and 4g of potassium persulfate into a beaker, then add 250mL of deionized water and stir until completely dissolved. Put the clean ITO glass vertically on the wall of the beaker, with the conductive surface facing the solution, and the non-conductive surface sealed with insulating glue. Then add 36 g of ammonia water with a mass concentration of 20%, take out the sample after stirring and reacting at 35° C. for 20 minutes, dry it naturally, and calcinate it under argon at 350° C. for 1.5 hours to prepare a porous nickel oxide film.
[0020] Weigh 1.6g of lithium perchlorate and dissolve it in 80g of acetonitrile solution, stir until completely dissolved. Then add 0.16 g of 3,4-ethylenedioxythiophene, stir and dissolve, and pass argon gas through the whole electrolyte for 30 minutes to remove oxygen. Then the electrolyte solution was transferred to an electrochemical three-electrode system, in which the porous nickel oxide film was used as th...
Embodiment 3
[0022] Weigh 64g of nickel sulfate and 8g of potassium persulfate into a beaker, then add 400mL of deionized water and stir until completely dissolved. Put the clean ITO glass vertically on the wall of the beaker, with the conductive surface facing the solution, and the non-conductive surface sealed with insulating glue. Then add 192g of ammonia water with a mass concentration of 20%, take out the sample after stirring and reacting at 35°C for 30 minutes, dry it naturally, and calcinate it under argon at 300°C for 2 hours to prepare a porous nickel oxide film.
[0023] Weigh 3.2g of lithium perchlorate and dissolve in 160g of acetonitrile solution, stir until completely dissolved. Then add 0.32 g of 3,4-ethylenedioxythiophene, stir and dissolve, and pass argon gas through the whole electrolytic solution for 30 minutes to remove oxygen. Then the electrolyte solution was transferred to an electrochemical three-electrode system, in which the porous nickel oxide film was used as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com