Bi-metal phosphide electrocatalyst as well as preparation method thereof and application thereof
A phosphide electric and bimetallic technology, applied in metal material coating process, electrode, electrolysis process, etc., to achieve the effect of lowering reaction barrier, improving electron transport and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
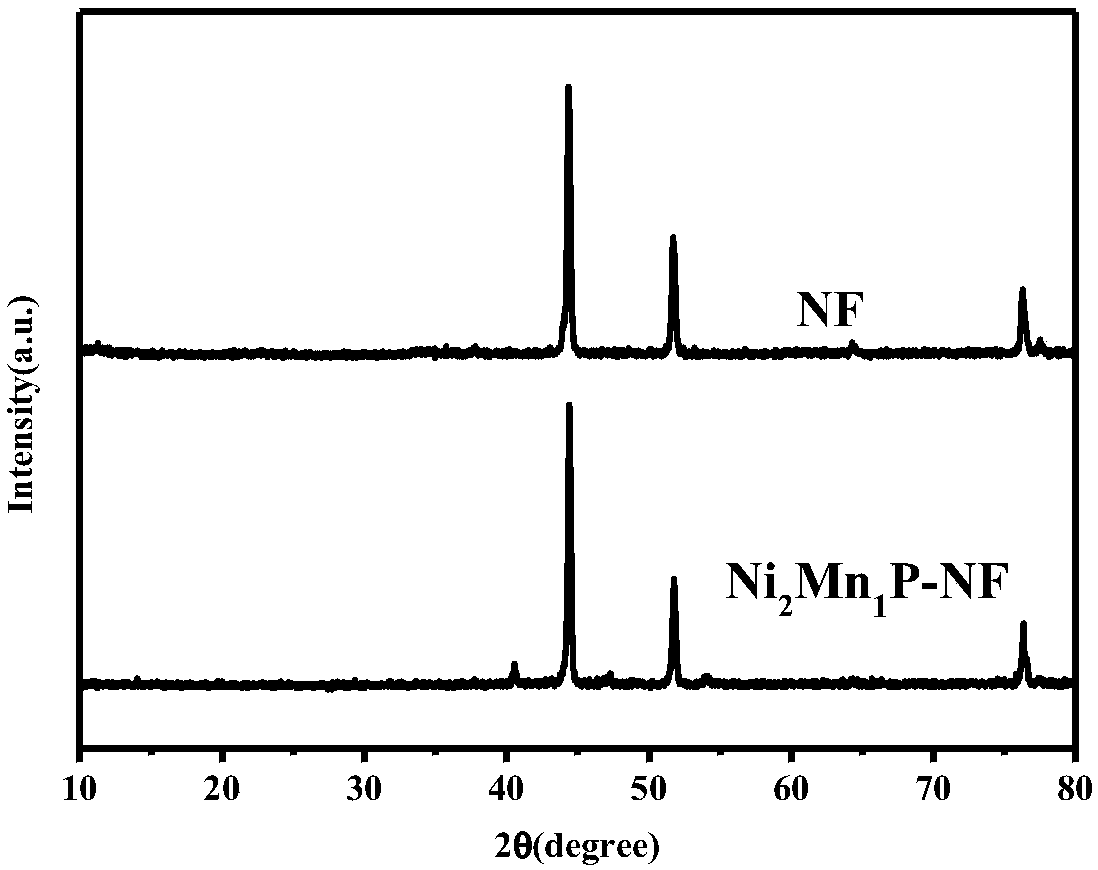
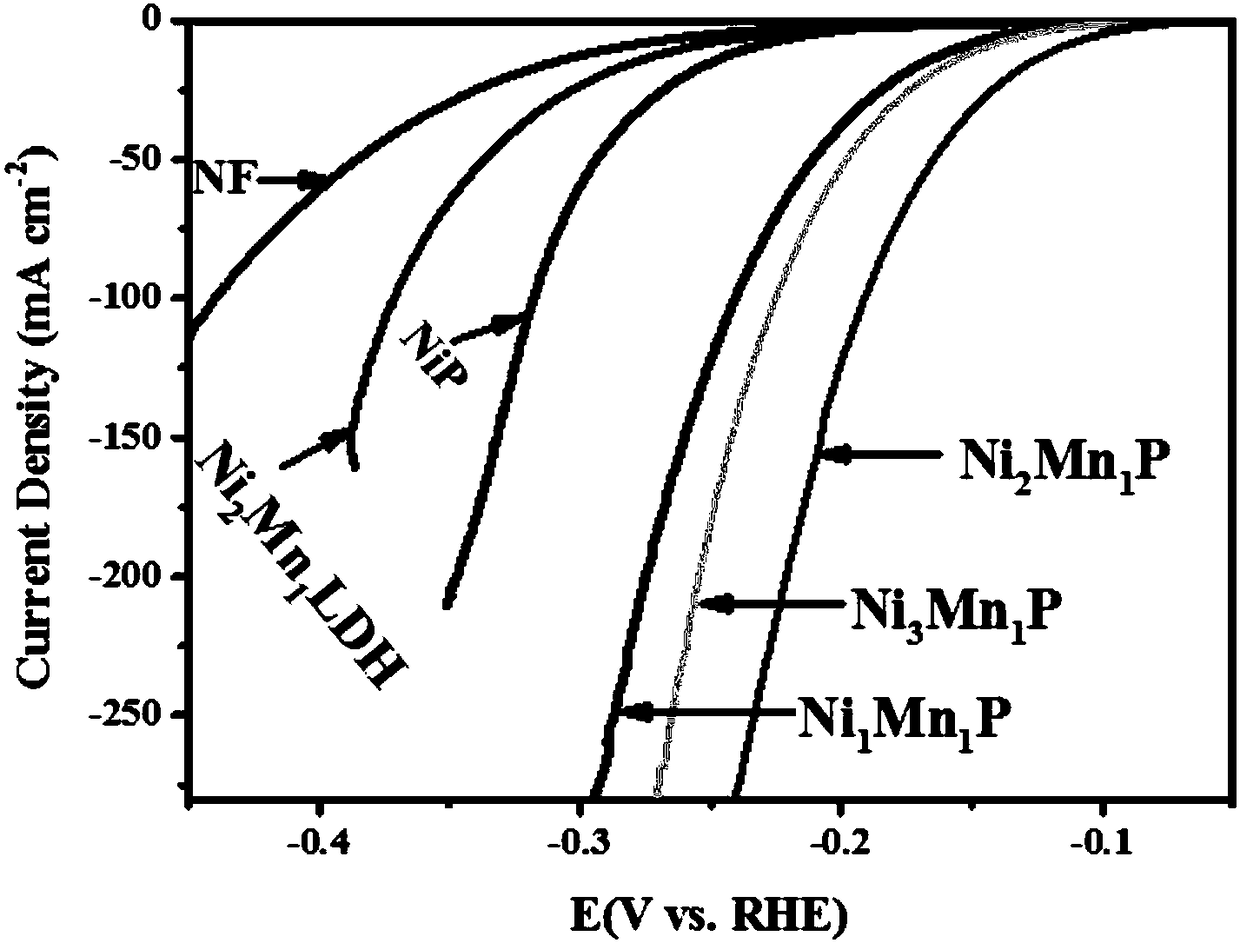
[0031] Nickel-manganese-phosphorus bimetallic phosphide (Ni 2 Mn 1 P-NF) preparation:
[0032] The nickel foam was ultrasonically cleaned with acetone, hydrochloric acid with a concentration of 3M, ethanol and deionized water for 30 minutes, and then dried at 60°C.
[0033] Weigh 0.118g NiCl 2 ·6H 2 O, and 0.0495g MnCl 2 ·4H 2 O, add 80mL deionized water to get precursor solution A (Ni:Mn=2:1); weigh out 0.0872g NaCl, 0.6308g HMT, 56.6μL H 2 O 2 (30w%) into solution A, stir for 30 minutes, put 2×1 (cm) foamed nickel into solution A, transfer to a 100mL reaction kettle, hydrothermally react at 100°C for 12h to obtain the khaki product NiMn-LDH -NF; Take out the foamed nickel, wash with water and alcohol, and dry.
[0034] Weigh NaH 2 PO 2 Place it on the upstream of the semi-closed crucible, place the NiMn-LDH-NF prepared above at the downstream of the semi-closed crucible, NaH 2 PO 2 The ratio of the amount to NiMn-LDH-NF is 5mol:1mol. The crucible is transferred to an automatically ...
Embodiment 2
[0036] The preparation method of the electrocatalytic material is basically the same as in Example 1, except that: NiCl 2 ·6H 2 The mass of O is 0.1783 g (Ni:Mn=3:1). The material is named Ni 3 Mn 1 P-NF.
Embodiment 3
[0038] The preparation method of the electrocatalytic material is basically the same as in Example 1, except that: NiCl 2 ·6H 2 The mass of O is 0.0594 g (Ni:Mn=1:1). The material is named Ni 1 Mn 1 P-NF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com