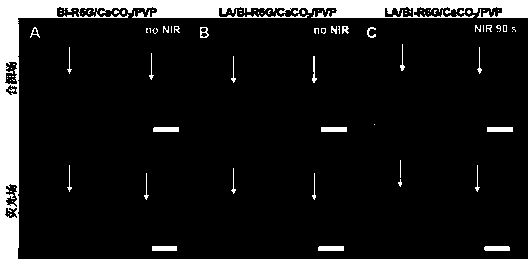
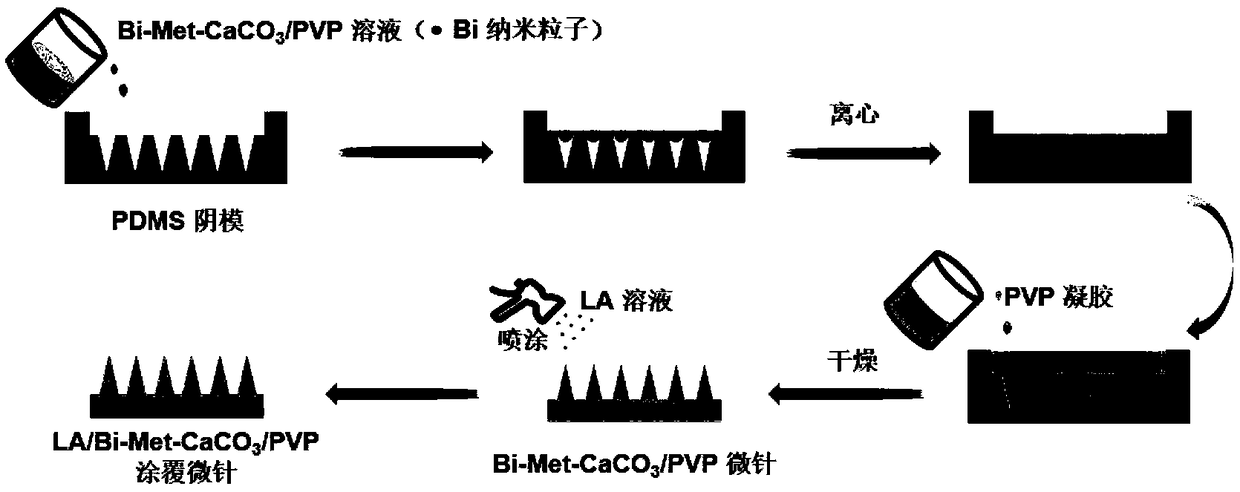
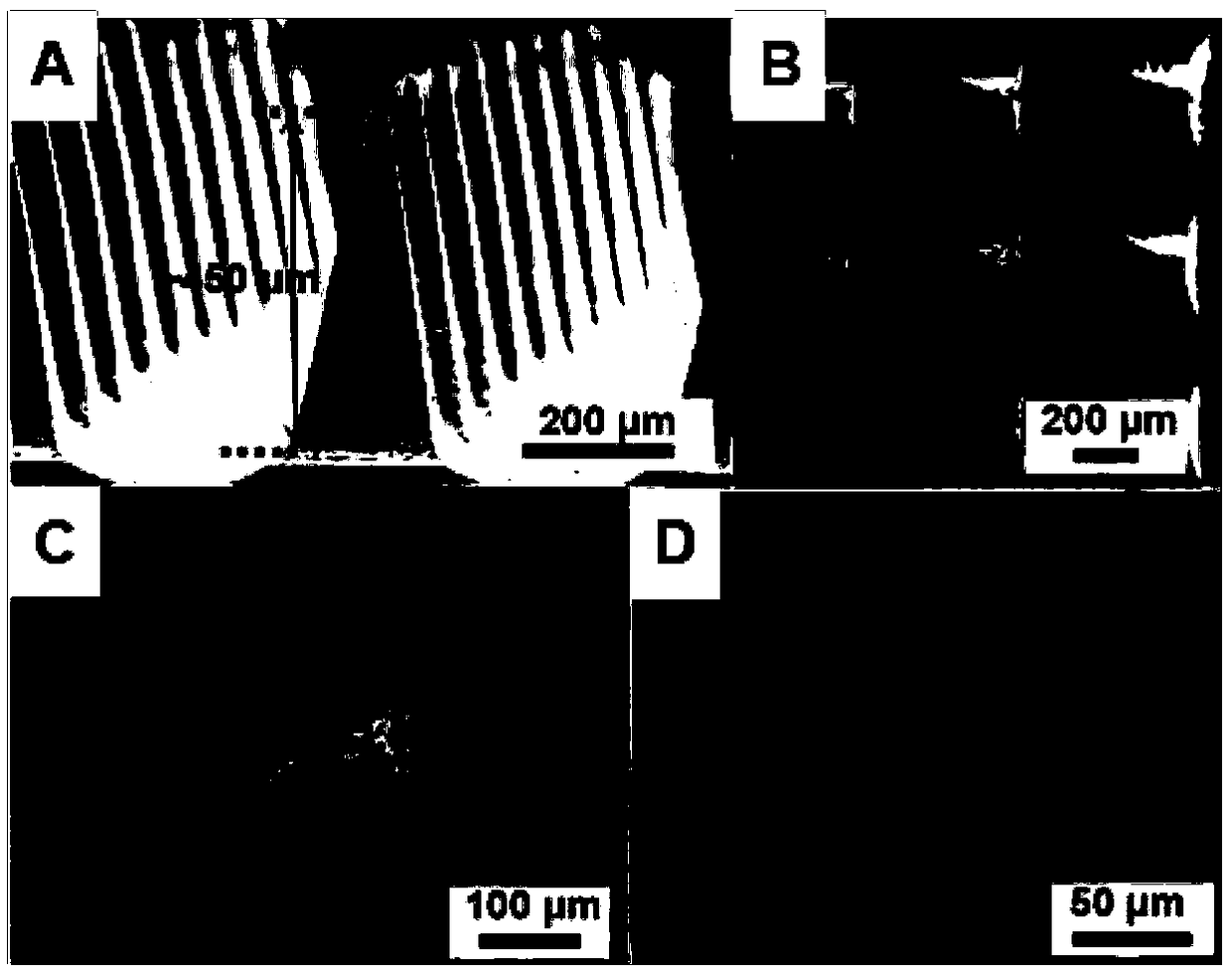
Photosensitive micro-needle, preparation method for same and controlled release method implemented by aid of photosensitive micro-needle
A micro-needle, photosensitive technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, metabolic diseases, etc., can solve the problems of short half-life, physical, mental and economic pressure of patients, long-term and frequent insulin injection, etc. Simple, easy to prepare in large batches, controlled release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Mix the liquid polydimethylsiloxane prepolymer (PDMS) and the curing agent at a mass ratio of 10:1, pour it on the PMMA microneedle male mold, then vacuumize to remove air bubbles, and then place it at normal pressure at 80°C Heating under ambient conditions for 6 hours, and finally demoulding to obtain a PDMS microneedle negative mold.
[0033] Get 0.5g polyvinylpyrrolidone (PVP) mixed with 0.1g calcium carbonate (CaCO 3 ), 0.3g metformin hydrochloride (Met) and 0.4g (0.044wt%) photothermal factor bismuth nano-quantum dots (Bi) were dissolved in 1mL deionized water and stirred evenly to obtain a needle mixture of drug-loaded microneedles. Wherein, the particle size of bismuth nano quantum dots is less than 10nm.
[0034] In addition, pure PVP hydrogel with a mass fraction of 40wt% needs to be configured as the microneedle matrix material.
[0035] Pipette 6 mg of the needle mixture of the drug-loaded microneedles and pour it into the cavity of the PDMS template, and ...
Embodiment 2
[0044] The preparation of the microneedle template is the same as in Example 1.
[0045] Get 0.6g polyvinylpyrrolidone (PVP) mixed with 0.05g calcium carbonate (CaCO 3 ), 0.3g metformin (Met) and 0.2g (0.022wt%) photothermal factor bismuth quantum dots (Bi) were dissolved in 1mL deionized water and stirred evenly to obtain a needle mixture of drug-loaded microneedles.
[0046] In addition, pure PVP hydrogel with a mass fraction of 30wt% needs to be configured as the microneedle matrix material.
[0047] Pipette 8 mg of the needle mixture of the drug-loaded microneedles and pour it into the cavity of the PDMS template, and then centrifuge at 8000 rpm for 10 min. Then pipette 30wt% PVP hydrogel and pour it into the cavity of the template again until it is completely filled, then centrifuge at 6000rpm for 10min, take out the whole mold and place it at room temperature to air dry naturally, and finally demould to obtain uncoated photosensitive microneedles Bi-Met / CaCO 3 / PVP.
...
Embodiment 3
[0050] The preparation of the microneedle template is the same as in Example 1.
[0051] Take 0.7g polyvinyl alcohol (PVA) mixed with 0.1g calcium carbonate (CaCO 3 ), 0.2g metformin and 0.8g (0.089wt%) photothermal factor bismuth quantum dots (Bi) were dissolved in 1mL deionized water and stirred evenly to obtain a needle mixture of drug-loaded microneedles.
[0052] In addition, pure PVA hydrogel with a mass fraction of 20 wt% needs to be configured as the microneedle matrix material.
[0053] Pipette 10 mg of the needle mixture of the drug-loaded microneedles and pour it into the cavity of the PDMS template, and then centrifuge at 8000 rpm for 8 min. Then pipette 20wt% PVA hydrogel and pour it into the inner cavity of the template again until it is completely filled, then centrifuge at 8000rpm for 10min, take out the whole mold and place it at room temperature to air dry naturally, and finally demould to obtain uncoated photosensitive microneedles Bi-Met / CaCO3 / PVP.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com