Core-shell nano drug for tumor in-situ visual therapy and preparation method thereof
A nano-drug, core-shell technology, applied in nano-drugs, anti-tumor drugs, preparations for in vivo tests, etc., can solve the problems of delayed treatment timing, low diagnosis and treatment efficiency, and long interval time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
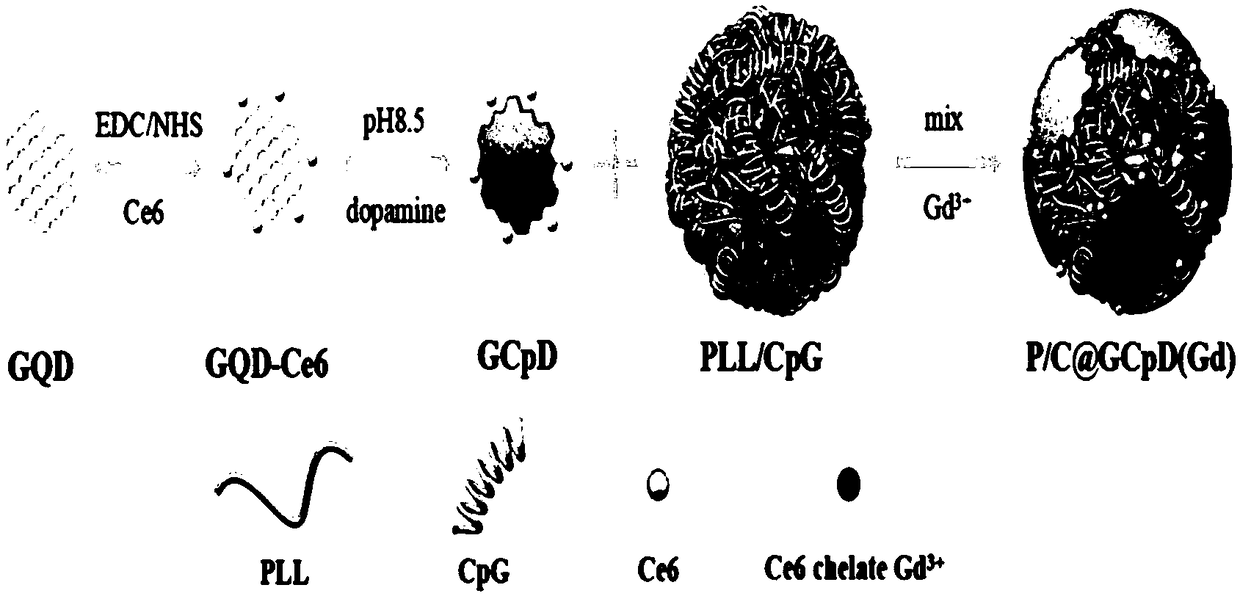
[0071] A core-shell nanomedicine for in situ visualization therapy of tumors, such as figure 1 As shown, it is characterized in that: a core-shell nano-medicine for in situ visualized treatment of tumors, characterized in that: the core of the core-shell nano-medicine includes: polycationic compound, and the polycationic compound passes through The immune adjuvant oligonucleotides combined by electrostatic attraction, the immune adjuvant oligonucleotides are labeled with fluorescent probes; the shell of the core-shell nano-medicine includes: a hydrophilic shell, and a hydrophilic shell An MRI imaging contrast agent chelated on the surface of the shell, the hydrophilic shell includes a negatively charged phototherapy drug modified by polydopamine, and the phototherapy drug is formed by copolymerization of aminated graphene quantum dots and a carboxyl-containing photosensitizer ; The core-shell structure is formed by electrostatic assembly between polycationic compounds and poly...
Embodiment 2
[0088] Stability experiment of nanomedicine:
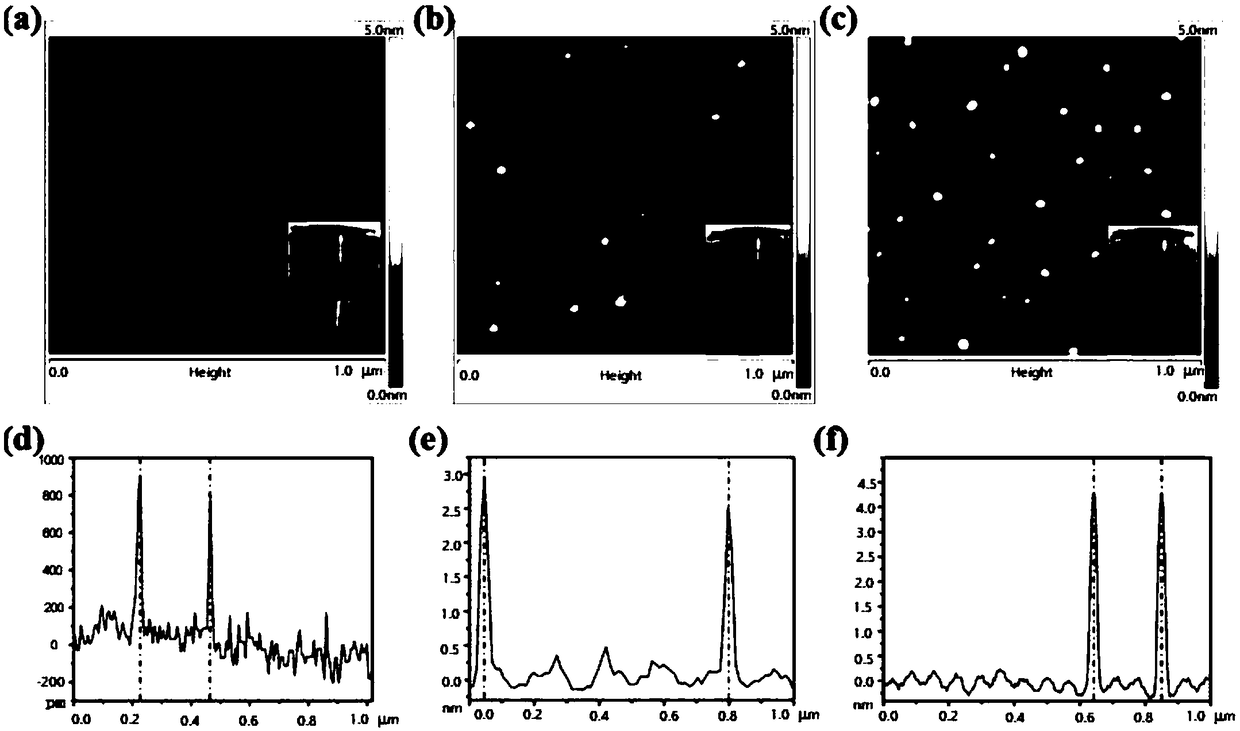
[0089] Such as Figure 5 As shown, figure (a) is the different incubation time of nano-medicine of the present invention and DNase I enzyme, and figure (b) is the protective effect of different nanoparticles on CpG, wherein M is Maker, 1 is free CpG, and 2 is CpG+DNase I Enzyme, 3 is P / C+DNase I enzyme+heparin sodium, 4 is P / C+heparin sodium+DNase I enzyme, 5 is P / C@GCpD(Gd)+DNase I enzyme+heparin sodium, 6 is P / C @GCpD(Gd) + sodium heparin + DNase I enzyme.
[0090] Through agarose gel electrophoresis experiments, it was shown that the P / C@GCpD(Gd) nanoparticles of the present invention can protect CpG from being degraded by enzymes. Compared with free CpG, the P / C@GCpD(Gd) particles of the present invention can Improve the circulation time of CpG in the body to a certain extent.
[0091] Such as Figure 6 As shown, figure (a) and figure (b) are respectively the change figure of particle size and PDI of P / C@GCpD (Gd) of the p...
Embodiment 3
[0093] In vitro photothermal and photodynamic properties experiments of nanomedicine:
[0094] Such as Figure 7 As shown, under 660nm laser excitation (1W / cm 2 , 10 minutes), the nanomedicine P / C@GCpD(Gd) of the present invention has a good photothermal heating effect measured by an infrared thermal imager and a thermocouple; P / C@GCpD(Gd) was measured by a singlet oxygen probe SOSG ( Gd) has a high singlet oxygen yield and can be used in photodynamic experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com