Preparation method of furfuran bio-based polyether ester copolymer and novel furfuran bio-based polyether ester copolymer
A technology of copolymer and polyether ester, which is applied in the field of furyl polyether ester synthesis, can solve the problems of high cost, reduced environmental significance, and loss, and achieve the effects of easy control, mild conditions, and good thermodynamic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
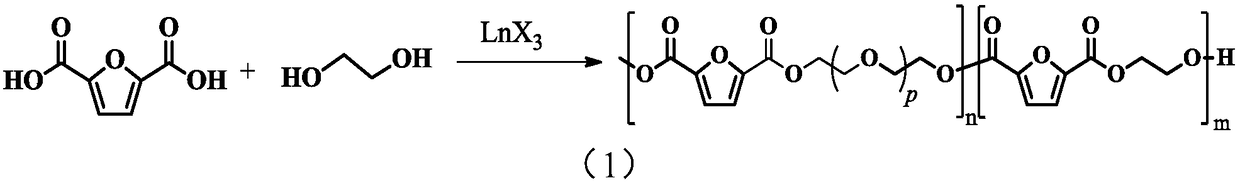
[0048] The invention provides a kind of preparation method of polyether ester copolymer, comprises the following steps:
[0049] 1) Under the conditions of a protective atmosphere and a metal complex catalyst, 2,5-furandicarboxylic acid and ethylene glycol undergo an esterification reaction, and then undergo a precondensation reaction and a polycondensation reaction to obtain a polyetherester copolymer.
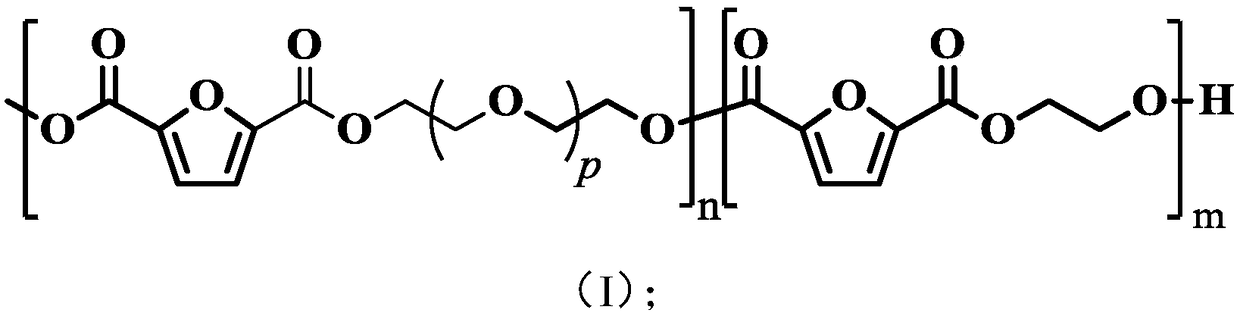
[0050] The present invention has no special restrictions on the structure of the polyether ester copolymer, the structure of the polyether ester copolymer well known to those skilled in the art can be used, and those skilled in the art can select according to actual production conditions, quality control and product requirements And adjustment, in the polyether ester copolymer of the present invention, the percentage content of the molar number of ethylene glycol segment accounts for the molar number of described polyether ester copolymer is preferably 30%~70%, that is more pr...
Embodiment 1
[0125] In the reactor, add 15.6 grams (0.1mol) of 2,5-furandicarboxylic acid, 18.0 grams (0.3mol) of ethylene glycol, and add 87 mg of samarium trifluoromethanesulfonate (accounting for the total amount of dicarboxylic acid monomers) 0.15% of 0.15%), under a nitrogen atmosphere, esterify at 180°C for 105 minutes to obtain an esterified product; Hours, yellow poly(ethylene glycol 2,5-furandicarboxylate-co-2,5-diethylene glycol furandicarboxylate-co-2,5-triethylene glycol furandicarboxylate) was obtained. for PPEGF.
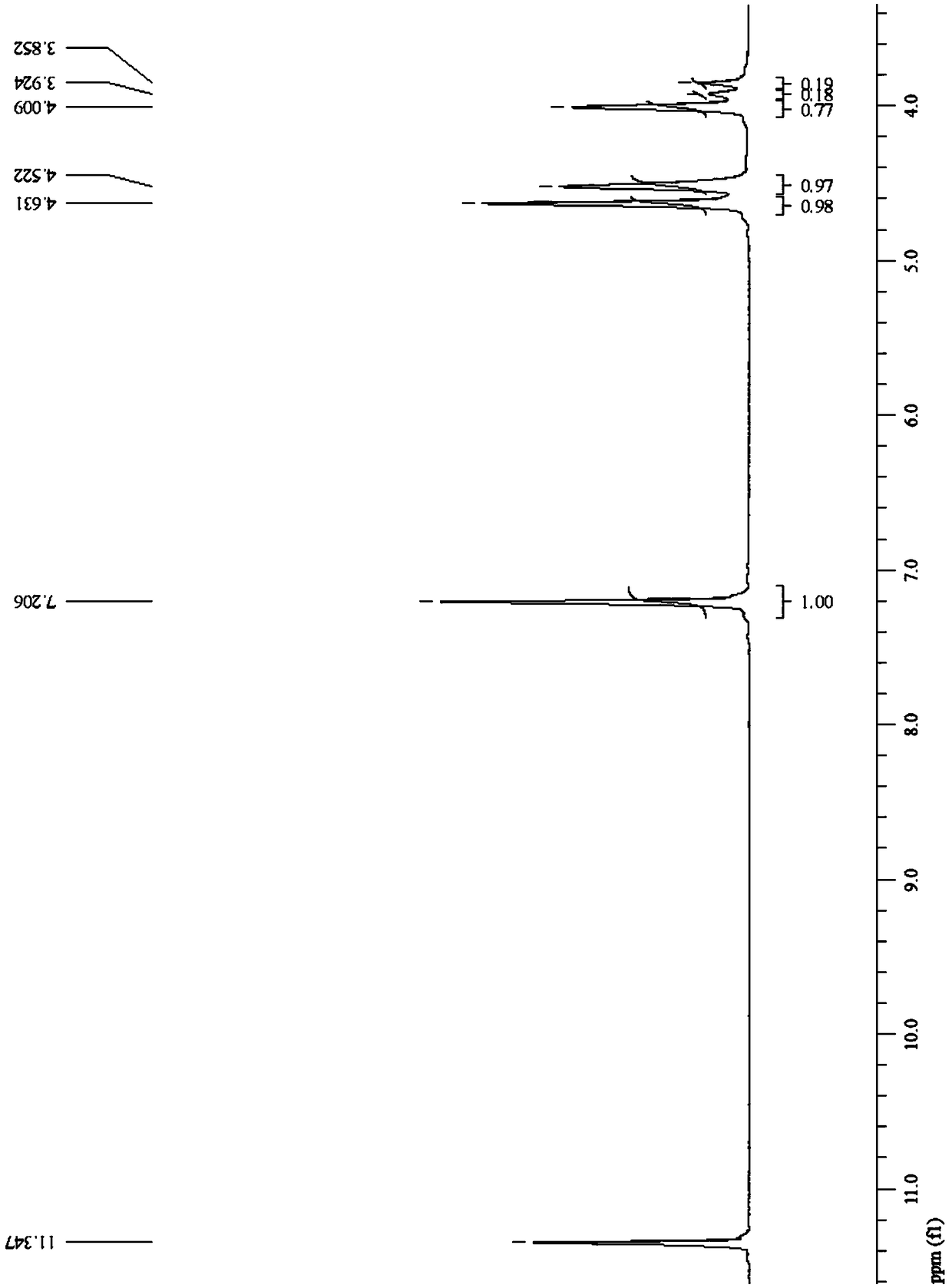
[0126] The furyl polyether ester copolymer prepared in Example 1 of the present invention was characterized, and the PPEGF copolyester was subjected to nuclear magnetic resonance analysis using deuterated trifluoroacetic acid as a solvent.
[0127] see figure 1 , figure 1 The proton nuclear magnetic resonance spectrum of the furanyl polyether ester copolyester prepared for Example 1 of the present invention. Depend on figure 1 It can be seen that the PPEGF cop...
Embodiment 2
[0135] In the reactor, add 15.6 grams (0.1mol) of 2,5-furandicarboxylic acid, 18.0 grams (0.3mol) of ethylene glycol, and add 72 milligrams of scandium trifluoromethanesulfonate (accounting for the total amount of dicarboxylic acid monomers) 0.15% of 0.15%), under a nitrogen atmosphere, esterify at 180°C for 105 minutes to obtain an esterified product; Hours, a yellow PPEGF copolymer was obtained.
[0136] Using deuterated trifluoroacetic acid as a solvent, the PPEGF copolyester is subjected to nuclear magnetic resonance analysis, the ratio of the ethylene glycol segment in the PPEGF copolyester structure to the glycol segment is 1:1, and the glycerol segment in the structure is Alcohol segments include diethylene glycol and triethylene glycol.
[0137] The PPEGF copolyester was dissolved in a mixed solvent of phenol and tetrachloroethane with a mass ratio of 1:1 at 25° C. to measure its intrinsic viscosity, and its intrinsic viscosity was 1.01 dL / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com