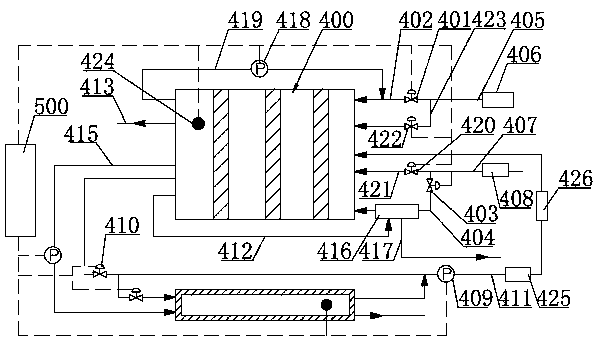
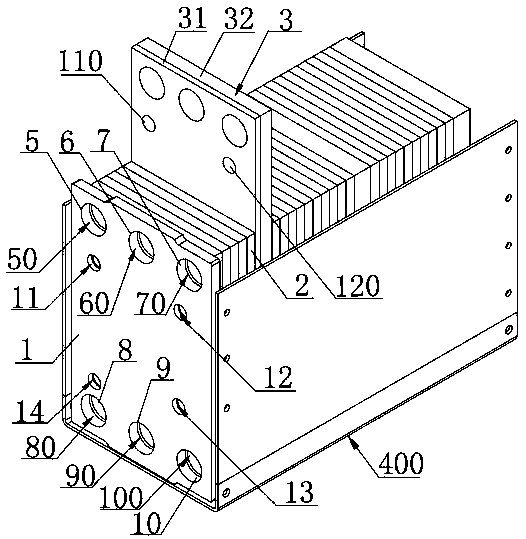
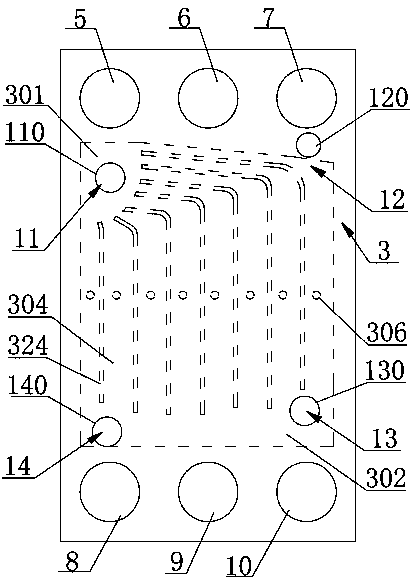
Low-temperature cold starting operation system for proton exchange membrane fuel cell
A proton exchange membrane, fuel cell technology, applied in fuel cells, fuel cell additives, fuel cell heat exchange, etc., can solve the problems of slow start, difficult start, insufficient reaction heat to dissolve ice, etc. The effect of a short cold start time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Environmental conditions: specific heat of graphite 710 J / (kg K); calorific value of hydrogen 1.4×10 8 J / kg; battery stack mass 200kg; ambient temperature -30°C; temperature after heating up 0°C; heat dissipation rate 5%.
[0036] Hydrogen consumption = (temperature after heating - ambient temperature) × specific heat of graphite × mass of battery stack ÷ calorific value of hydrogen × (1+ heat dissipation rate).
[0037]Hydrogen consumption=30×710×200÷(1.4×10 8 )×1.05=0.032kg.
example 2
[0039] Environmental conditions: ambient temperature -20°C; temperature after heating up 0°C; hydrogen consumption flow rate 0.048kg / min; graphite specific heat 710 J / (kg K); hydrogen calorific value 1.4×10 8 J / kg; battery stack mass 200kg; heat dissipation rate 5%.
[0040] Among them: the hydrogen consumption flow is determined according to the hydrogen supply capacity of the hydrogen supply system for the fuel cell system, and the working hydrogen consumption under the rated power of the fuel cell is determined, taking a 36kw fuel cell as an example.
[0041] Hydrogen consumption = (temperature after heating - ambient temperature) × specific heat of graphite × mass of battery stack ÷ calorific value of hydrogen × (1+ heat dissipation rate).
[0042] Hydrogen consumption=20×710×200÷(1.4×10 8 )×1.05=0.022kg.
[0043] Cold start time = hydrogen consumption ÷ hydrogen flow.
[0044] Cold start time=0.022÷0.048=0.46 min=28 s.
[0045] That is: from the ambient temperature -...
example 3
[0047] Environmental conditions: ambient temperature -10°C; temperature after heating up 0°C; hydrogen consumption flow rate 0.048kg / min; graphite specific heat 710 J / (kg K); hydrogen calorific value 1.4×10 8 J / kg; battery stack mass 200kg; heat dissipation rate 5%.
[0048] Among them: the hydrogen consumption flow is determined according to the hydrogen supply capacity of the hydrogen supply system for the fuel cell system, and the working hydrogen consumption under the rated power of the fuel cell is determined, taking a 36kw fuel cell as an example.
[0049] Hydrogen consumption = (temperature after heating - ambient temperature) × specific heat of graphite × mass of battery stack ÷ calorific value of hydrogen × (1+ heat dissipation rate).
[0050] Hydrogen consumption=10×710×200÷(1.4×10 8 )×1.05=0.011kg.
[0051] Cold start time = hydrogen consumption ÷ hydrogen flow.
[0052] Cold start time=0.011÷0.048=0.23 min=14 s;
[0053] That is: from the ambient temperature -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com