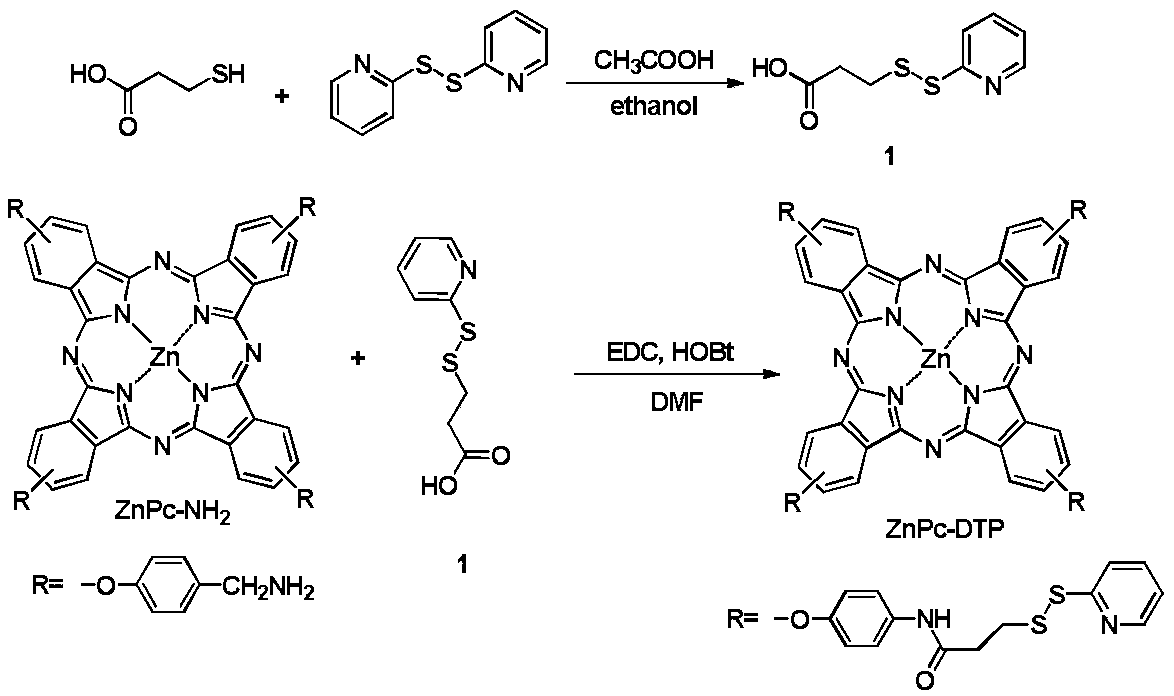
Dithiopyridine modified zinc phthalocyanine as well as preparation method and application thereof
A technology of dithiobipyridine and dithiobipyridine, which is applied in the field of biopharmaceuticals, can solve the problems of reducing the effect of PDT on tumor treatment, limiting the wide application of PDT therapy, and insufficient tumor targeting selectivity, so as to increase the selective retention capacity, Reduced antioxidant capacity, effect of highly effective antitumor therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] After 3-mercaptopropionic acid (0.53g) and 2,2'-dithiodipyridine (2.2g) are uniformly mixed in ethanol (5mL), glacial acetic acid (300uL) is added, and the reaction is stirred at room temperature for 8h to obtain Intermediate 1;
[0040] Intermediate 1 (121mg) was dissolved in dichloromethane (0.5mL), EDC (87.67mg) and HOBt (87.67mg) were added to catalyze 2h, ZnPc-NH 2 (50mg) dissolved in N,N-dimethylformamide (4mL) was added to the reaction system, then 39mg potassium carbonate was added, and the coupling reaction was carried out in an ice-water bath for 24h to obtain ZnPc-DTP, which was used to prepare dithiobipyridine modified phthalocyanine derivatives Flow chart as figure 2 Shown.
Embodiment 2
[0042] After 3-mercaptopropionic acid (0.53g) and 2,2'-dithiopyridine (1.65g) are uniformly mixed in ethanol (10mL), glacial acetic acid (300uL) is added, and the reaction is stirred at room temperature for 8 hours to obtain Intermediate 1;
[0043] Intermediate 1 (101.3mg) was dissolved in dichloromethane (0.5mL), EDC (73.06mg) and HOBt (63.59mg) were added to catalyze 4h, ZnPc-NH 2 (50mg) dissolved in N,N-dimethylformamide (4mL) was added to the reaction system, then 52mg of potassium carbonate was added, and the coupling reaction was carried out in an ice-water bath for 48h to obtain ZnPc-DTP.
Embodiment 3
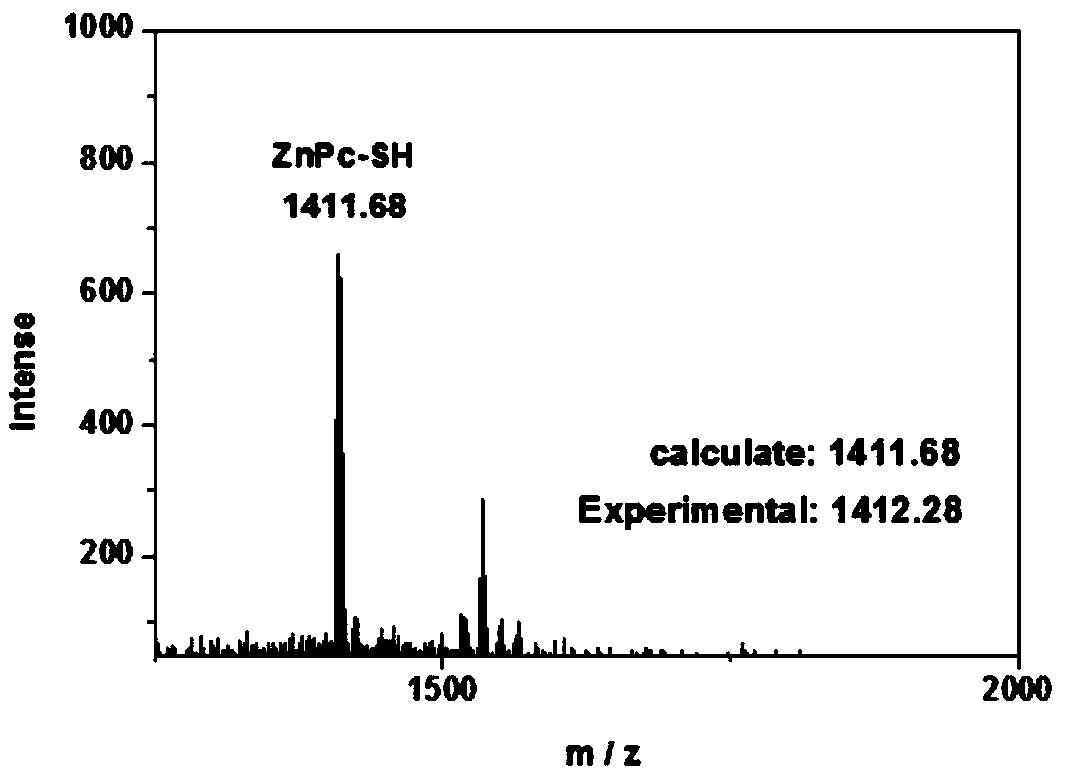
[0045] Monitoring the reaction of ZnPc-DTP and GSH under physiological conditions
[0046] The ZnPc-DTP prepared in Example 1 was reacted with GSH under physiological conditions, and the product ZnPc-SH was verified by high-resolution mass spectrometry. The molar ratio of the reaction between ZnPc-DTP and GSH is 1:80, and triethylamine is added to make the solution weakly alkaline (pH=7.4), and react for 24 hours at room temperature. After the product is separated and purified, it is determined to be ZnPc-SH by high resolution mass spectrometry.
[0047] Such as image 3 As shown, the theoretically calculated molecular weight of ZnPc-DTP is 1411.68, and the actual measured value is 1412.28, indicating that the product is ZnPc-SH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com