Nanometer gel preparation with dual slow release effect and preparation method thereof
A nano-gel, sustained-release technology, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, capsule delivery, etc. Change the problem of low ability to achieve the effect of benefiting the body's absorption, high encapsulation rate, and promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides a nanogel preparation with double sustained release, which is prepared from the following raw materials in weight percentage:
[0046] Paclitaxel 10%
[0047] Liquid crystal material 40% (phospholipid: glycerol: co-solvent = 6: 1: 0.5)
[0048] Rheology modifier 50%
[0049] The preparation method of the nanogel preparation with double sustained release is as follows:
[0050] S1 Weigh phosphatidylcholine, diolein, ethanol and 10% paclitaxel in a weight ratio of 6:1:0.5 according to 40% of the total weight and mix and stir for 30 minutes;
[0051] S2 adding a small amount of water to the precursor solution obtained in S1 for high-shear dispersion, and then performing high-pressure homogenization of the obtained mixture to obtain drug-loaded liquid crystal nanoparticles;
[0052] S3, then add 50% of the total weight of poloxamer 407 to the mixed solution of S2, stir slowly for 2 hours, and control the temperature at about 4°C, so that the syst...
Embodiment 2
[0055] This embodiment is basically the same as Embodiment 1, the difference lies in the following aspects:
[0056] A nanogel preparation with double sustained release and a preparation method thereof, prepared from the following raw materials in percentage by weight:
[0057] Paclitaxel 10%
[0058] Liquid crystal material 40% (phospholipid: glycerol: co-solvent = 10: 4: 1.5)
[0059] Rheology modifier 50%
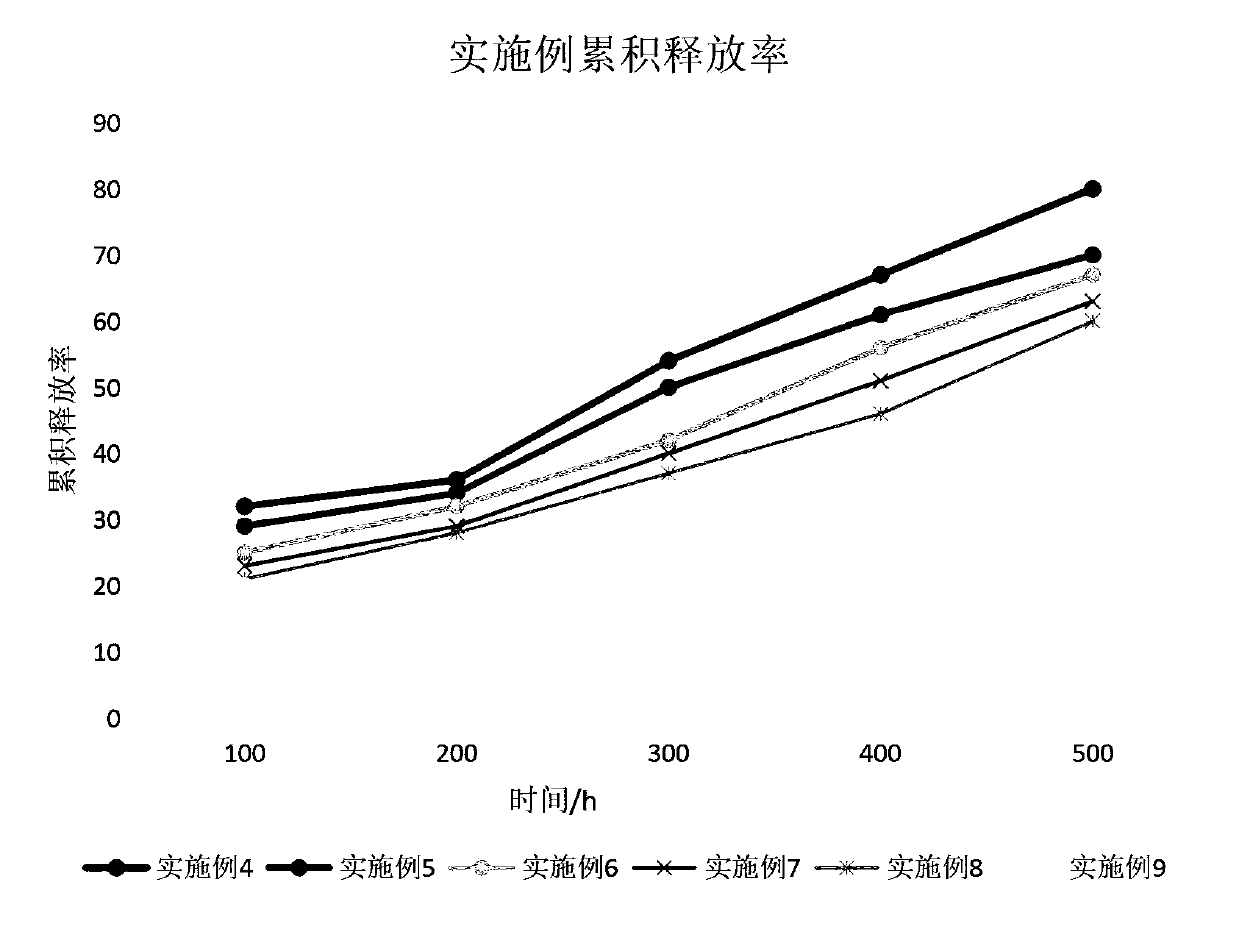
[0060] The release efficiency of the nanogel preparation prepared in this example with double sustained release was investigated, and the encapsulation rate was 82%, and the cumulative release rate within one week was 33%.
Embodiment 3
[0062] This embodiment is basically the same as Embodiment 1, the difference lies in the following aspects:
[0063] A nanogel preparation with double sustained release and a preparation method thereof, prepared from the following raw materials in percentage by weight:
[0064] Paclitaxel 10%
[0065] Liquid crystal material 40% (phospholipid: glycerol: co-solvent = 8: 2: 1)
[0066] Rheology modifier 50%
[0067] The release efficiency of the nanogel preparation prepared in this example with double sustained release was investigated, and the encapsulation rate was 86%, and the cumulative release rate within one week was 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com