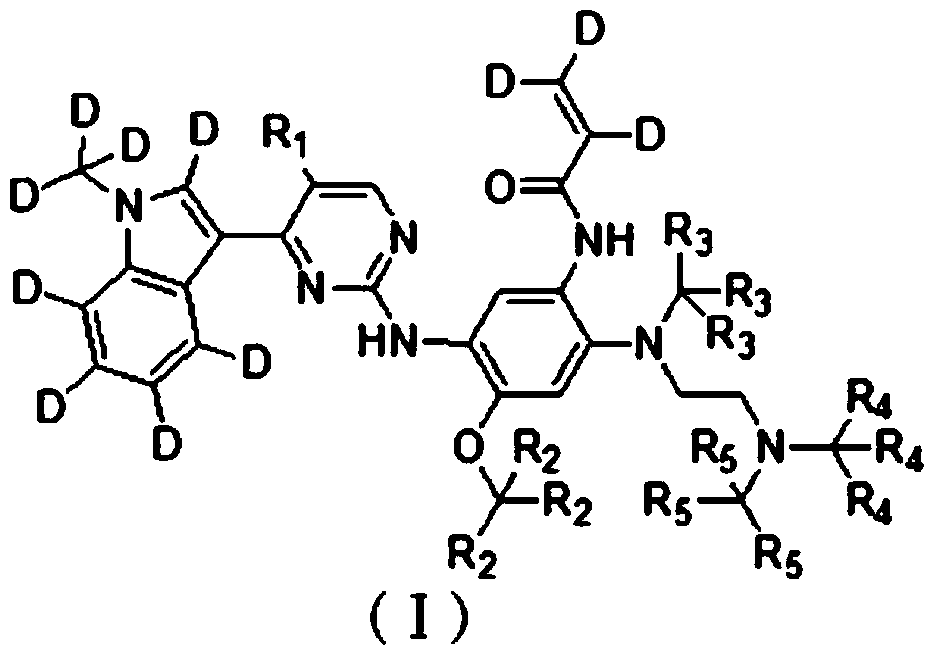
Deuterated pyrimidine compound, preparation method, pharmaceutical composition, preparation and application
A technology of deuterated pyrimidine and pharmaceutical preparations, which can be used in drug combination, organic chemistry, anti-tumor drugs, etc., and can solve the problems of instability, toxic and side effects of AZD9291
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
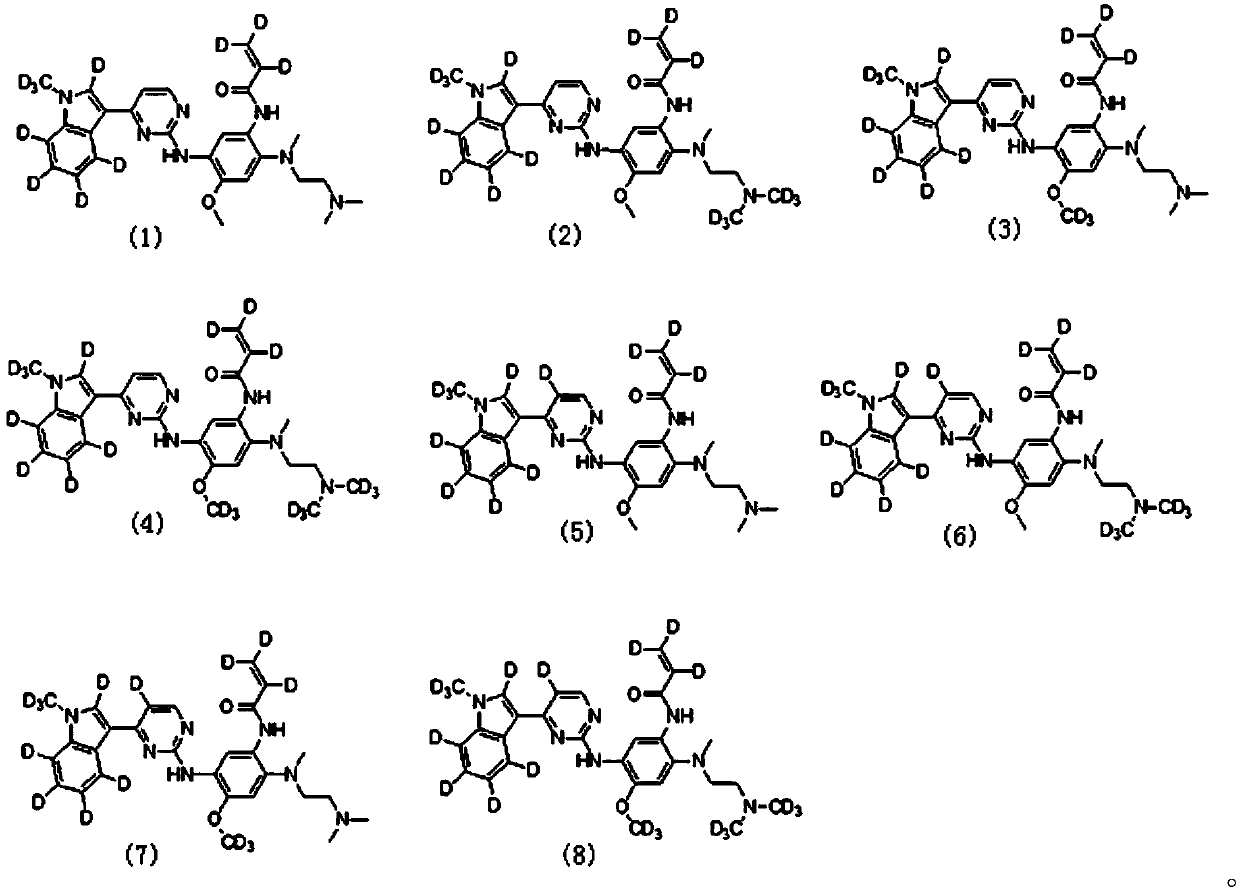
[0083] The preparation of embodiment 1 formula (1) compound
[0084]
[0085] 1. Synthesis of intermediate a
[0086]
[0087] Under the protection of argon, add indole-d7 (3g, 24mmol) and 20mL anhydrous THF (tetrahydrofuran) to a 100mL three-necked flask successively, cool down to 0°C, and add sodium hydride (NaH) (60%, dispersed in Mineral oil) (1.4g, 36mmol), stirred and reacted at 0°C for 30min, then added deuterated iodomethane (4.2g, 29mmol) dropwise, heated to room temperature and reacted for about 3 hours. After the reaction, cool to 0°C, add 20 mL of saturated NH4Cl aqueous solution to the reaction mixture, extract with ether (30 mL×3), combine the organic phases, wash once with saturated brine, dry over anhydrous sodium sulfate, and spin dry to obtain a crude product.
[0088] The obtained crude product was subjected to silica gel column chromatography (eluent: petroleum ether (PE): ethyl acetate (EA) = 100:0-90:10), and the obtained eluent was spin-dried to o...
Embodiment 2
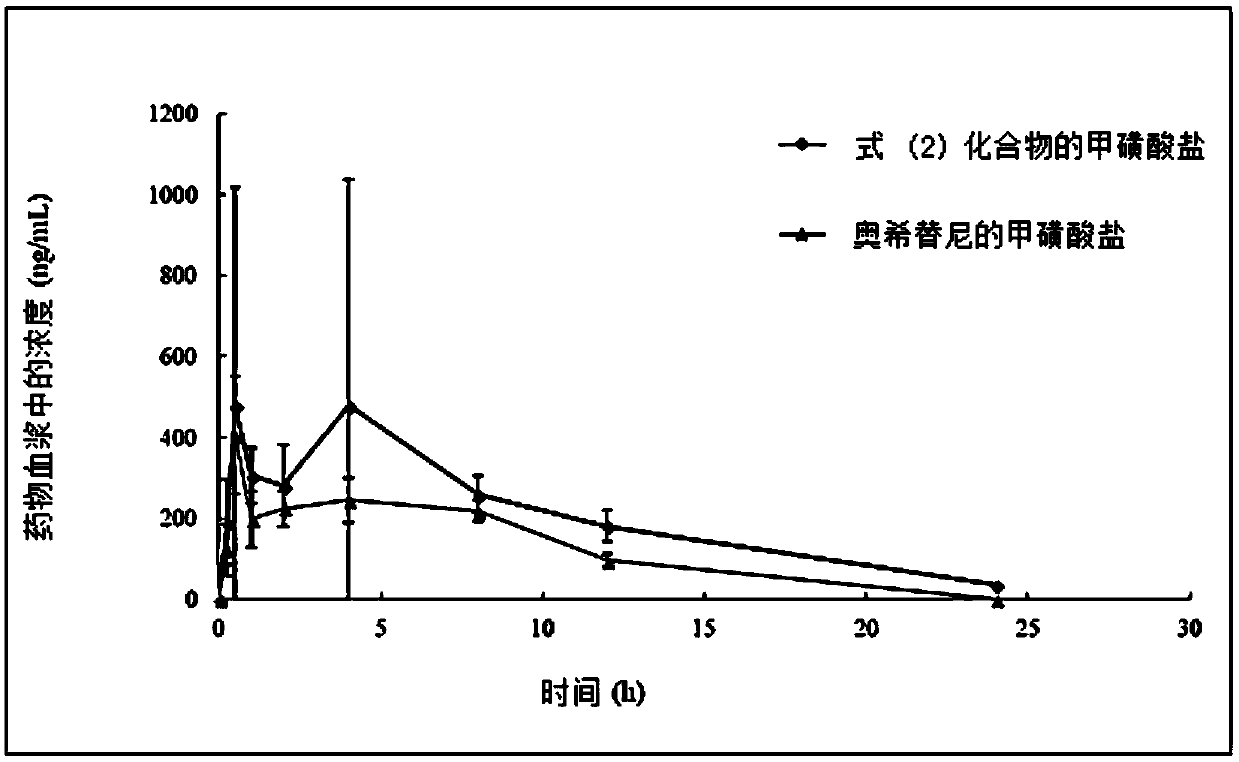
[0109] The preparation of embodiment 2 formula (2) compound
[0110]
[0111] When preparing the compound of formula (2), in addition to replacing the compound of formula (C1) (N, N'-trimethylethylenediamine) in step 4 in the above example 1 with the compound of formula (C2) (N1 , N1-bis(methyl-d3)-N2-methyl-1,2-diamine), other steps refer to Example 1.
[0112] The prepared compound is a light yellow solid. LC-MS: 516.9.
[0113]
[0114] A hydrogen NMR data is: 1 H-NMR: (300MHz, DMSO-d 6 ,ppm)2.30(m,2H),2.73(s,3H),2.90(m,2H),3.87(s,3H),7.05(s,1H),7.23(m,1H),7.91(s,1H ), 8.33(m,1H), 9.15(s,1H), 10.22(s,1H). It is proved that the obtained compound is the compound of formula (2).
Embodiment 3
[0115] The preparation of embodiment 3 formula (3) compound
[0116]
[0117] When preparing the compound of formula (3), in addition to replacing the compound of formula (B1) (4-fluoro-2-methoxy-5-nitroaniline) in step 3 in the above-mentioned embodiment 1 with the following formula (B2 ) compound (4-fluoro-2-(methoxy-d3)-5-nitroaniline), other steps refer to Example 1. The prepared compound is a light yellow solid. LC-MS: 513.9.
[0118]
[0119] A hydrogen NMR data is: 1 H-NMR: (300MHz, DMSO-d 6 , ppm) 2.22 (s, 6H), 2.31 (m, 2H), 2.73 (s, 3H), 2.90 (m, 2H), 7.05 (s, 1H), 7.23 (m, 1H), 7.91 (s, 1H ), 8.33 (m, 1H), 9.15 (s, 1H), 10.22 (s, 1H). It is proved that the obtained compound is the compound of formula (3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com