Decolorizing peroxidase and preparation method and application thereof
A technology of peroxidase and tryptophan, which is applied in the field of artificial metalloenzymes, can solve the problems of long catalysis time and achieve the effects of simple operation, increased catalytic conversion number, and reduced dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
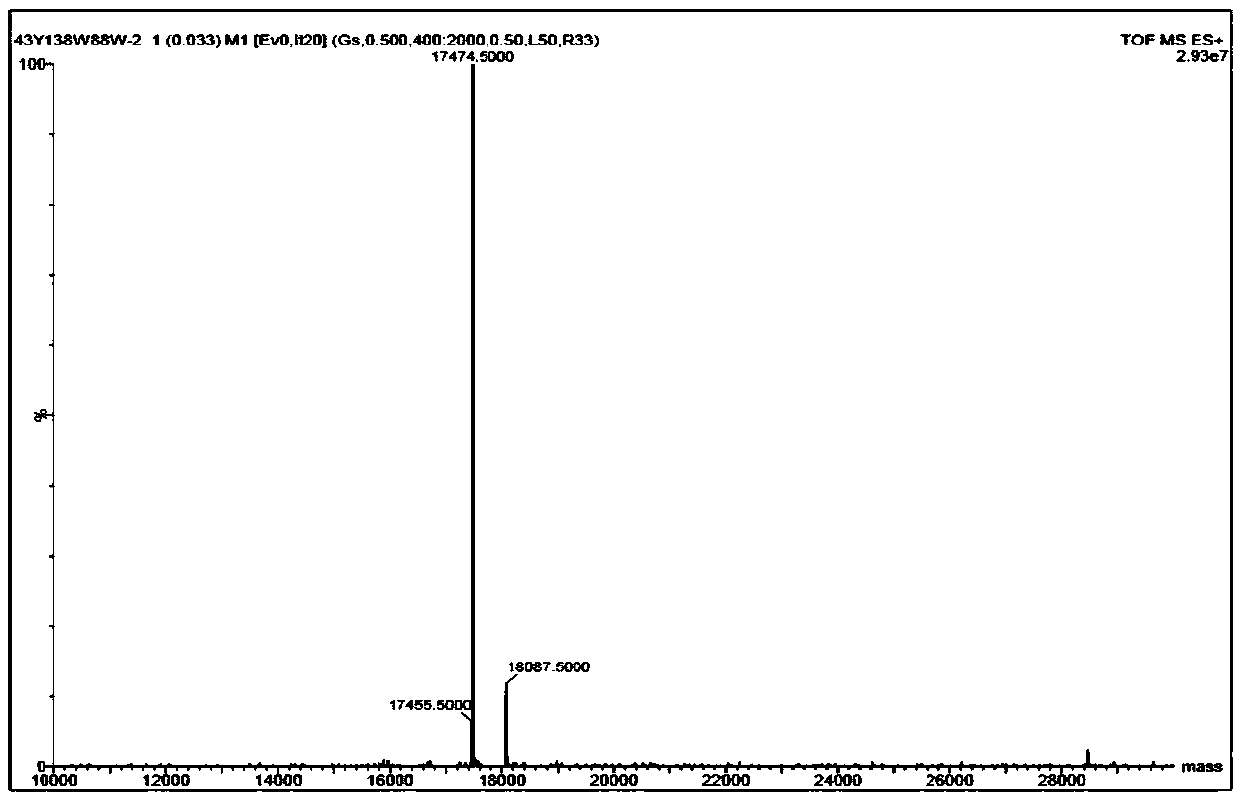
[0034]Based on genetic engineering and protein engineering, using site-directed mutation technology, tyrosine was introduced at the 43rd position near the heme active center of myoglobin, tryptophan was introduced at the 138th position, and tryptophan was introduced at the 88th position outside the heme active center at the same time, Furthermore, it was expressed in Escherichia coli BL21(DE3), and separated and purified by ion exchange column (DEAE 52 resin) and gel column (Superdex75) to obtain F43Y / F138W / P88W Mb mutant protein. The protein molecular weight was shown by mass spectrometry ( figure 1 ).
[0035] The amino acid sequence (SEQ ID NO.1) of the F43Y / F138W / P88W Mb mutant is as follows:
[0036] VLSEGEWQLVLHVWAKVEADVAGHGQDILIRLFKSHPETLEKYDRFKHLKTEAEMKASEDLKKHGVTVLTALGAILKKKGHHEAELKWLAQSHATKHKIPIKYLEFISEAIIHVLHSRHPGDFGADAQGAMNKALELWRKDIAAKYKELGYQG.
Embodiment 2
[0038] 7 μM F43Y / F138W / P88W Mb was prepared in 100 mM phosphate buffer (pH 7.0). At the same time, prepare 10mM H with deionized water 2 o 2 and 10mM active blue substance RB19 (Reactive blue, RB19) mother solution, the concentration is calibrated with ultraviolet spectrum (H 2 o 2 for ε 240 nm=39.4m -1 cm -1 , RB19 is ε 595 nm=10mM -1 cm -1 ).
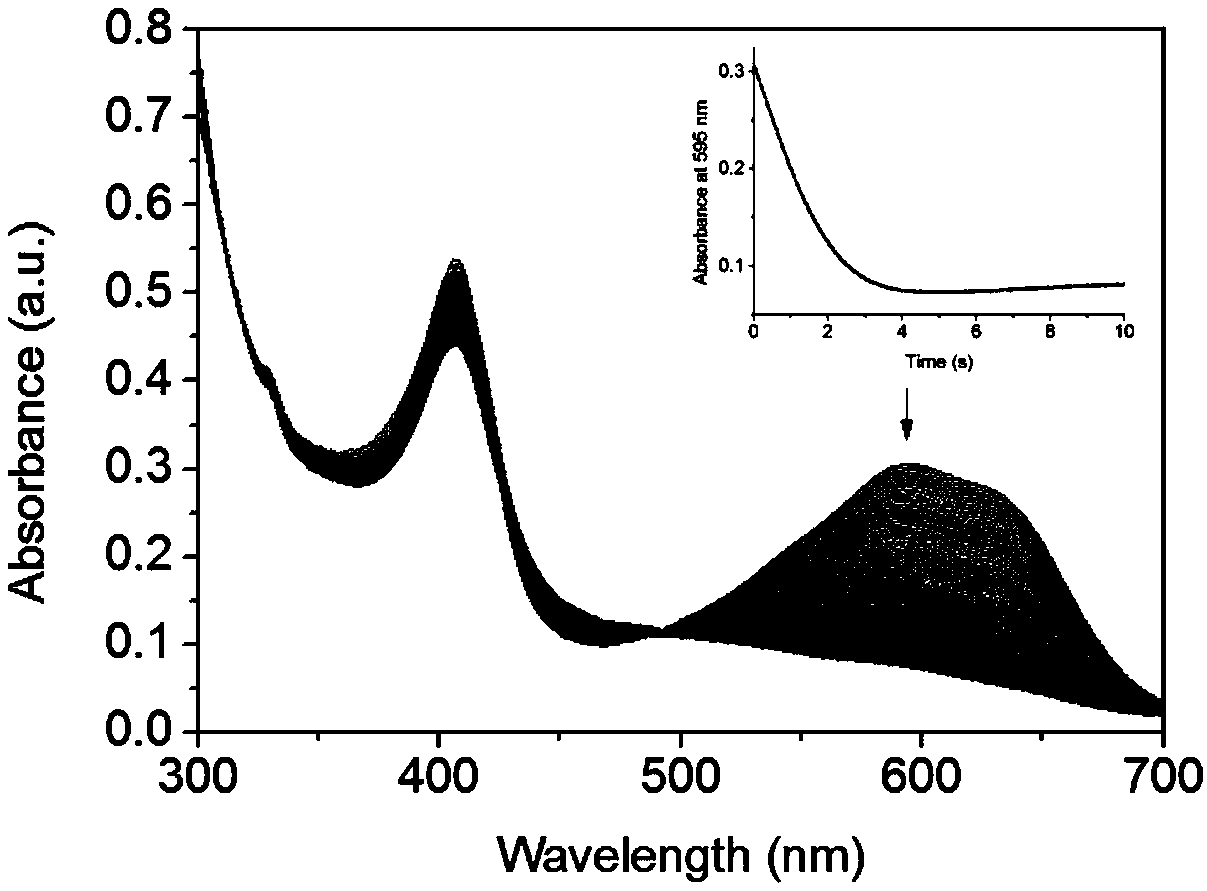
[0039] Take 2 mL of the above F43Y / F138W / P88W Mb solution, add 20 μL of 10 mM RB19 mother solution, mix evenly and place it in the injector C of the rapid dwell spectrometer, and take 10 mM H 2 o 2 2mL was placed in the D injector. The catalytic reaction was carried out after sample injection and mixing by C and D injectors, the reaction time was 10s, and a total of 500 spectra were collected ( figure 2 ). Monitor the change of RB19 characteristic absorption 595nm absorption peak with time ( figure 2 illustration).
[0040] The results showed that F43Y / F138W / P88W Mb could make the absorption peak of dye RB19 at 595nm...
Embodiment 3
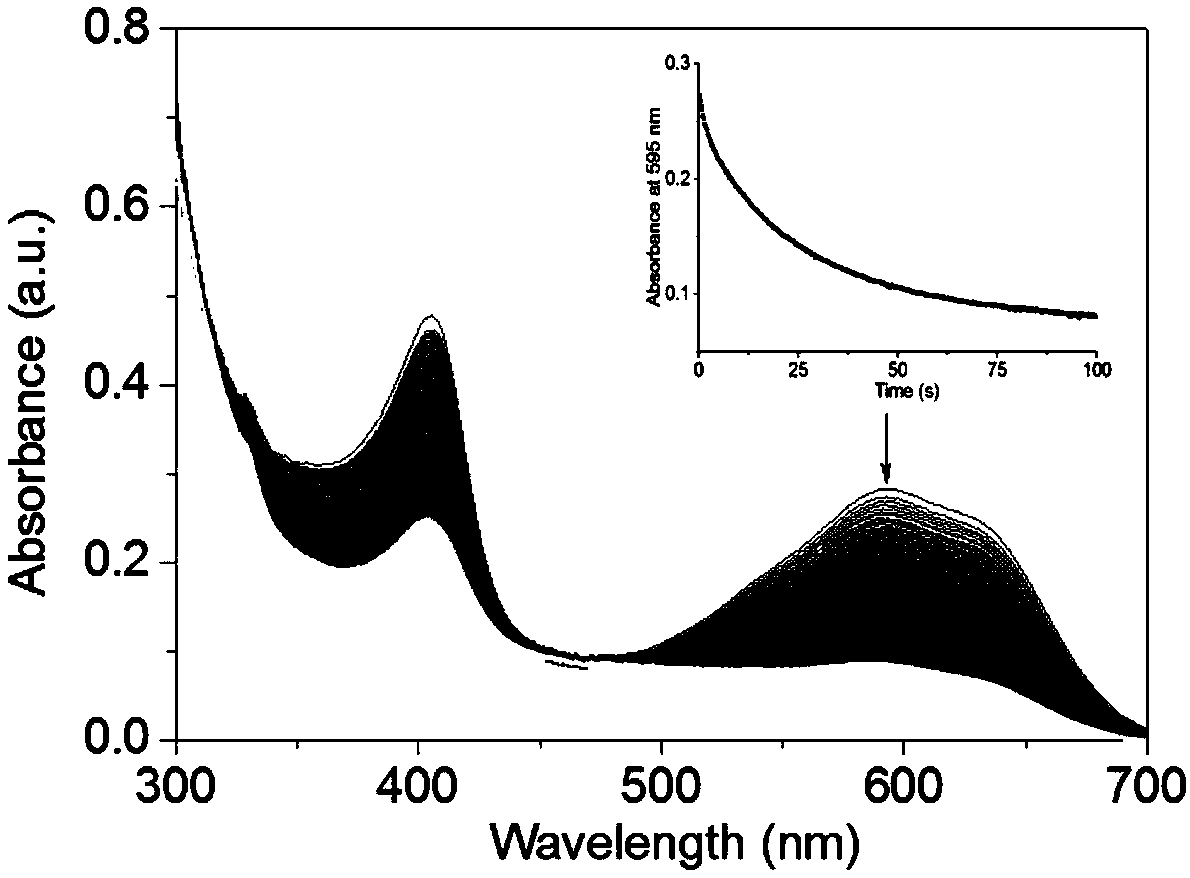
[0042] 7 μM F43Y / F138W / P88W Mb was prepared in 100 mM phosphate buffer (pH 7.0). At the same time, prepare 10mM H with deionized water 2 o 2 and 10mM active blue substance RB19 mother solution (Reactive blue, RB19), the concentration is calibrated with ultraviolet spectrum (H 2 o 2 for ε 240 nm=39.4m -1 cm -1 , RB19 is ε 595 nm=10mM -1 cm -1 ).
[0043] Take two cuvettes of the same specification (clean and dry), add 1mL of the above F43Y / F138W / P88W Mb solution to each, and add 10μL of 10mM RB19 mother solution and mix well. Add 1mL 100mM phosphate buffer to one of the cuvettes, and add 1mL 10mM H to the other cuvette 2 o 2 , the operation requires simultaneous execution. React for 5s and take pictures quickly. The color of the solution before and after the reaction is as follows: image 3 .
[0044] The results showed that F43Y / F138W / P88W Mb could decolorize the dye RB19 within a few seconds (5s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com