Detection method of genotoxic impurity diisopropyl sulfate in medicine
A technology of diisopropyl sulfate and a detection method, which is applied in the field of analysis and testing, can solve the problems of low detection limit, increase the difficulty of measuring the content of drug impurities, complex drug raw material matrix, etc., so as to improve the recovery rate and the pretreatment rate. , improve sensitivity and accuracy, reduce the effect of matrix interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Establishment of detection method
[0054] (1) Sample pretreatment
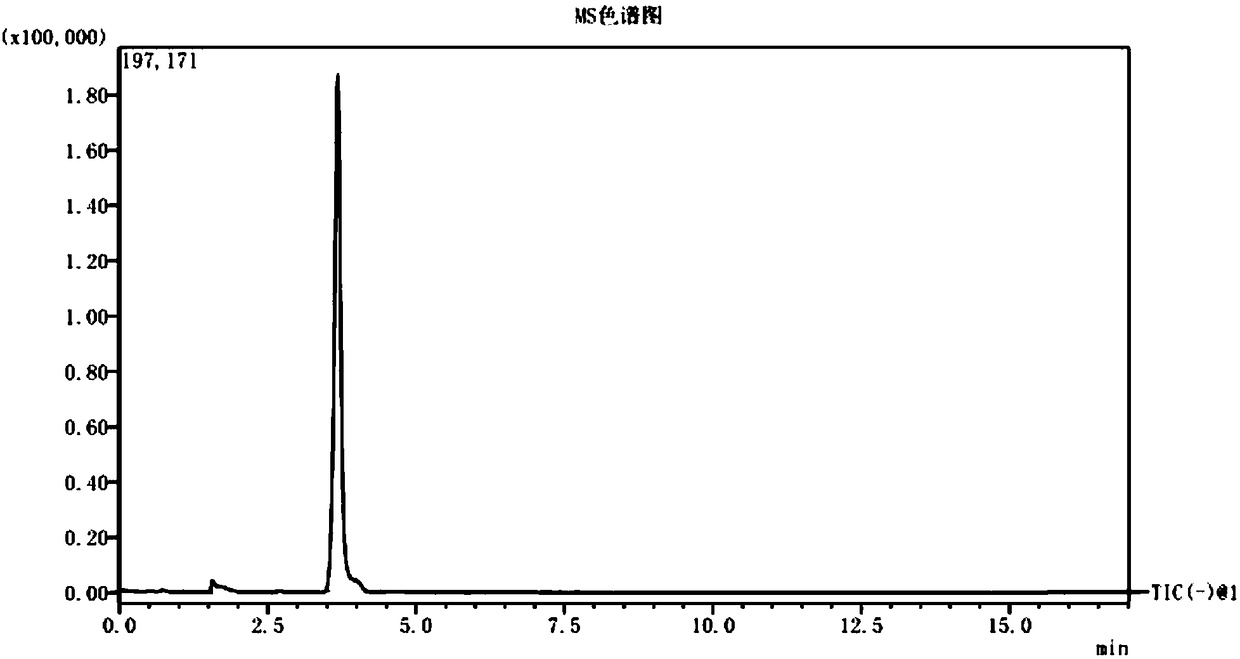
[0055] Precisely weigh 0.1g±0.01g of thymol raw material, add 1mL of methanol to extract, vortex and centrifuge at 5000r / min for 10min. The supernatant is passed through a 0.45μm microporous membrane, and then the supernatant is subjected to triple series connection. Grade rod liquid mass spectrometry (LC-MS / MS) analysis.
[0056] (2) Preparation of standard working solution
[0057] Accurately weigh 10.0 mg of diisopropyl sulfate, dissolve it with methanol to make the volume to 100 mL, and mix well to obtain a 100 μg / mL diisopropyl sulfonate reference substance stock solution. Precisely measure 100μL of the diisopropyl sulfonate reference substance stock solution, dilute to 10mL with methanol, and mix well to obtain a 1μg / mL standard reference substance solution. Take 1μg / mL standard reference solution 10μL, 50μL, 200μL, 100μL, 200μL, 500μL, 1000μL, add methanol to dilute to 1.0mL, and obtain the ...
Embodiment 2
[0086] Example 2 Detection of actual samples
[0087] Precisely weigh 0.1013g (Sample 1) and 0.1008g (Sample 2) of thymol APIs respectively into two centrifuge tubes, add 1.0mL methanol to each, and centrifuge at 5000r / min after vortexing. After separation for 10 minutes, the supernatant was passed through a 0.45 μm microporous filter membrane, and 5.0 μL of the liquid was taken for HPLC-tandem mass spectrometry detection according to the method established in Example 1. The detection results are shown in Table 6.
[0088] Table 6 Content of Diisopropyl Sulfate in Thymol Pharmaceutical Raw Materials
[0089] sample name
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com