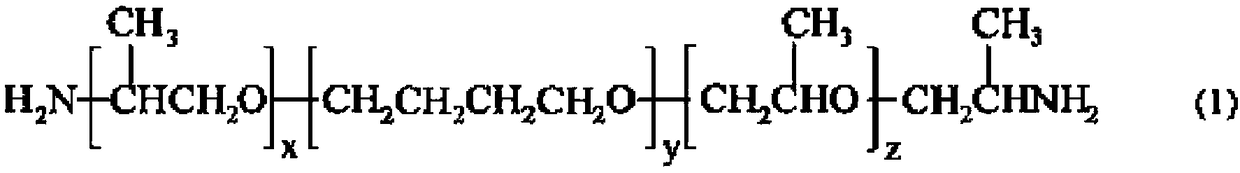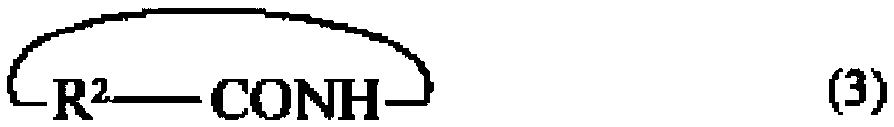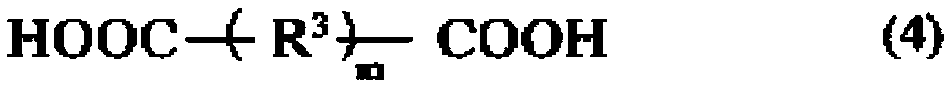Polyamide elastomer composition, and fiber and molded article comprising same
A polyamide elastomer, lactam compound technology, applied in the direction of one-component polyamide rayon, textiles and papermaking, fasteners, etc., can solve the problems of easy dye shedding, poor dye reactivity, low weather resistance, etc. The effect of excellent dyeing fastness and excellent dyeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Polyamide Elastomer Manufacturing Process
[0163] Put 12-aminododecanoic acid (manufactured by Ube Industries, Ltd.) 17.54 into a pressure vessel with a capacity of 70 liters equipped with a stirrer, a thermometer, a torque gauge, a pressure gauge, a nitrogen inlet, a pressure regulator, and a polymer outlet. kg, adipic acid (manufactured by Asahi Kasei Co., Ltd.) 0.30 kg, XYX type triblock polyether diamine (manufactured by HUNTSMAN: ELASTAMINE RT-1000) 2.09 kg, Irganox 245 (manufactured by BASF JAPAN) 0.06 kg, and phosphorous acid (manufactured by Taihei Chemical Industry Co., Ltd.) 0.004 kg. After the inside of the container was fully replaced with nitrogen, the pressure in the container was adjusted to 0.05 MPa while supplying nitrogen gas at 200 liters / hour, and the temperature in the container was raised from room temperature to 230° C. over 1 hour, and the pressure in the container was maintained at 0.05 while performing polymerization at 230°C.
[0164] The a...
Embodiment 2~4、 comparative example 2、3
[0172] In the polyamide elastomer manufacturing process of Example 1, the amount of phosphorous acid charged was changed as shown in the table below, and 250 minutes after the temperature in the tank reached 230°C was regarded as the polymerization end point. Polymerized to obtain pellets.
[0173] At this time, the stirring current value at the polymerization start point was subtracted from the stirring current value at the polymerization end point to check the degree of increase in the stirring current value. In general, as the degree of polymerization increases, the stirring current value also tends to increase, so it is used as an index for controlling the degree of polymerization. The results are shown in Table 1.
[0174] Next, spinning was performed by the same method as in the "spinning step" described in Example 1 using the obtained pellets.
[0175] [Table 1]
[0176]
[0177] In Comparative Example 1, although the degree of polymerization also increased and pe...
Embodiment 5~6、 comparative example 4
[0180] Dyeability was confirmed using the difference in the amount of amino terminal groups. Comparative Example 4 A polyamide elastomer was obtained in the same manner as in Example 1, except that the amount of adipic acid added in the polyamide elastomer manufacturing process was 0.39 kg. In Example 5, a polyamide elastomer was obtained by the same method as in Example 1. In Example 6, a polyamide elastomer was obtained as in Example 1 except that the amount of ELASTAMINE RT-1000 added was 3.00 kg. Furthermore, according to the spinning step of Example 1, the polyamide elastomer obtained above was melt spun to obtain polyamide elastomer fibers. The obtained fiber was used to produce a tube knitted fabric, and the dyeability and color fastness were evaluated according to the above-mentioned evaluation method. The evaluation results are shown in Table 2.
[0181] [Table 2]
[0182]
[0183] When the color fastness was evaluated according to "5. Color fastness", the colo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com