25mg amitriptyline thin-film coating tablet prescription and technological procedure
A technology of amitriptyline and film coating, applied in the field of medicine, can solve the problems of difficulty in decomposing, regulating the mood of patients, and unfavorable absorption by patients, and achieve the effects of improving taste, improving mood, and being easy to decompose and absorb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
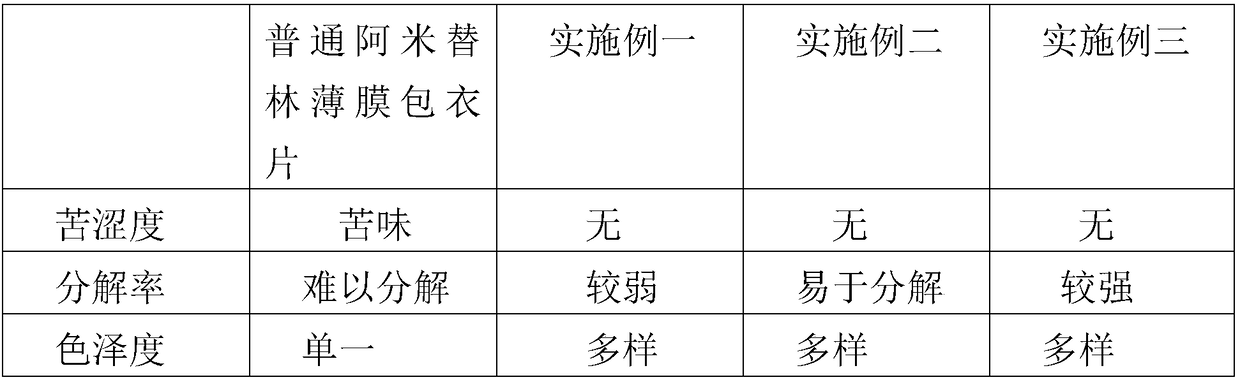
[0032] A 25mg amitriptyline film-coated tablet prescription is characterized in that: it is made of the following components by weight, tablet core: 84 parts by weight of calcium hydrogen phosphate dihydrate, 1 part by weight of cornstarch, 15 parts by weight of purified water, 20 parts by weight of amitriptyline hydrochloride, 5 parts by weight of lactose, 9 parts by weight of corn starch, 6 parts by weight of microcrystalline cellulose, 0.8 parts by weight of anhydrous silica gel, 0.5 parts by weight of stearic acid, and 0.2 parts by weight of magnesium stearate, Coating: 5 parts by weight of coating powder, 25 parts by weight of isopropanol, and 25 parts by weight of purified water.
[0033] A kind of technological specification of 25mg amitriptyline film-coated tablet prescription, comprises the following steps,
[0034] (1), lactose, cornstarch, microcrystalline cellulose, anhydrous silica gel, stearic acid, magnesium stearate, amitriptyline hydrochloride, calcium hydroge...
example 2
[0052] A 25mg amitriptyline film-coated tablet prescription is characterized in that: it is made of the following components by weight, tablet core: 85.5 parts by weight of calcium hydrogen phosphate dihydrate, 2 parts by weight of cornstarch, 15.5 parts by weight of purified water, 25 parts by weight of amitriptyline hydrochloride, 6 parts by weight of lactose, 10 parts by weight of corn starch, 6.5 parts by weight of microcrystalline cellulose, 1.3 parts by weight of anhydrous silica gel, 1 part by weight of stearic acid, 0.5 parts by weight of magnesium stearate, Coating: 5.5 parts by weight of coating powder, 30 parts by weight of isopropanol, and 30 parts by weight of purified water.
[0053] A kind of technological specification of 25mg amitriptyline film-coated tablet prescription, comprises the following steps,
[0054] (1), lactose, cornstarch, microcrystalline cellulose, anhydrous silica gel, stearic acid, magnesium stearate, amitriptyline hydrochloride, calcium hydr...
example 3
[0072] A 25mg amitriptyline film-coated tablet prescription is characterized in that: it is made of the following components by weight, tablet core: 87 parts by weight of calcium hydrogen phosphate dihydrate, 3 parts by weight of cornstarch, 16 parts by weight of purified water, 30 parts by weight of amitriptyline hydrochloride, 7 parts by weight of lactose, 11 parts by weight of cornstarch, 7 parts by weight of microcrystalline cellulose, 1.8 parts by weight of anhydrous silica gel, 1.5 parts by weight of stearic acid, 0.8 parts by weight of magnesium stearate, Coating: 6 parts by weight of coating powder, 35 parts by weight of isopropanol, and 35 parts by weight of purified water.
[0073] A kind of technological specification of 25mg amitriptyline film-coated tablet prescription, comprises the following steps,
[0074] (1), lactose, cornstarch, microcrystalline cellulose, anhydrous silica gel, stearic acid, magnesium stearate, amitriptyline hydrochloride, calcium hydrogen p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com