Development and application of a class of hole-transporting polymer materials based on carbazole-anthracene structure
A polymer material and hole transport technology, applied in the field of organic electronics, can solve the problems of low carrier mobility, difficult energy level adjustment, poor stability, etc., and achieve high carrier mobility, good Solution processability, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
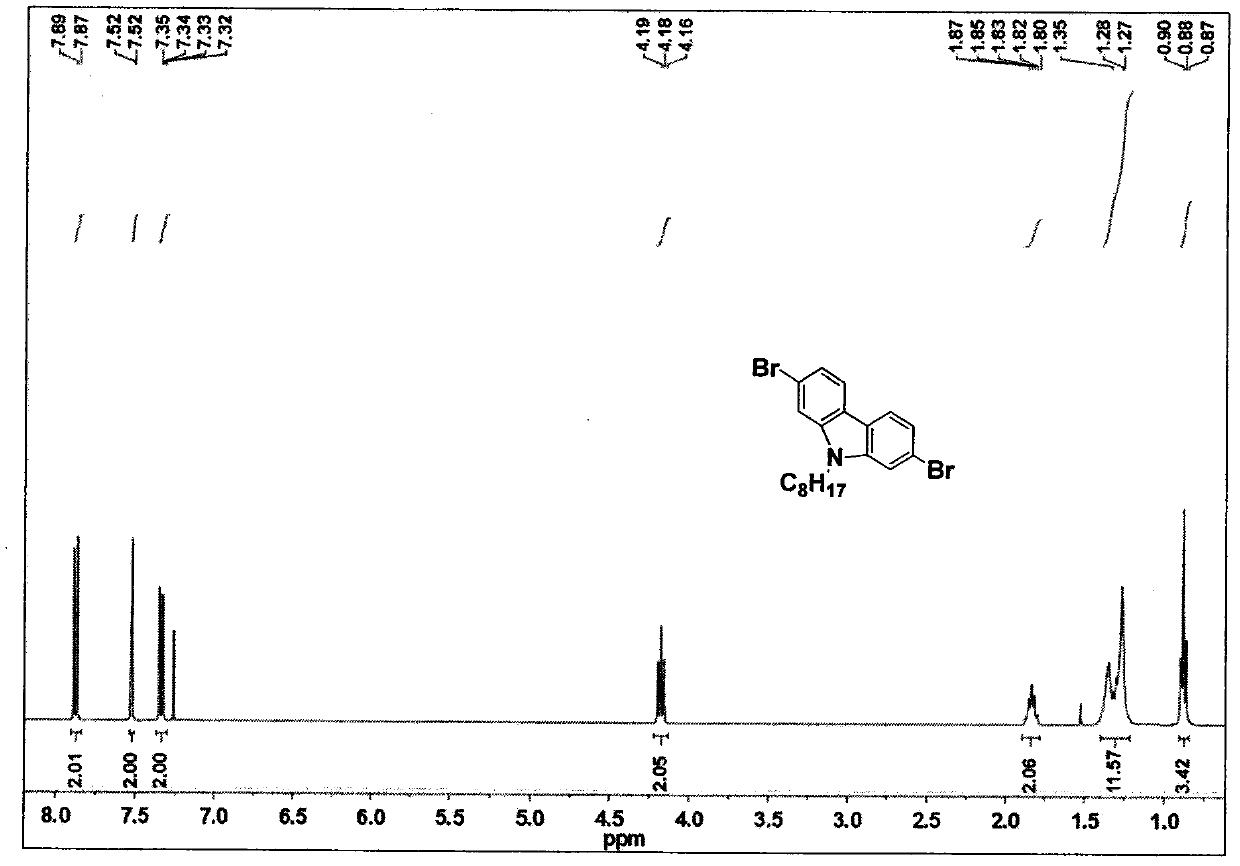
example 1
[0038] (1) Synthesis of monomer M1
[0039]
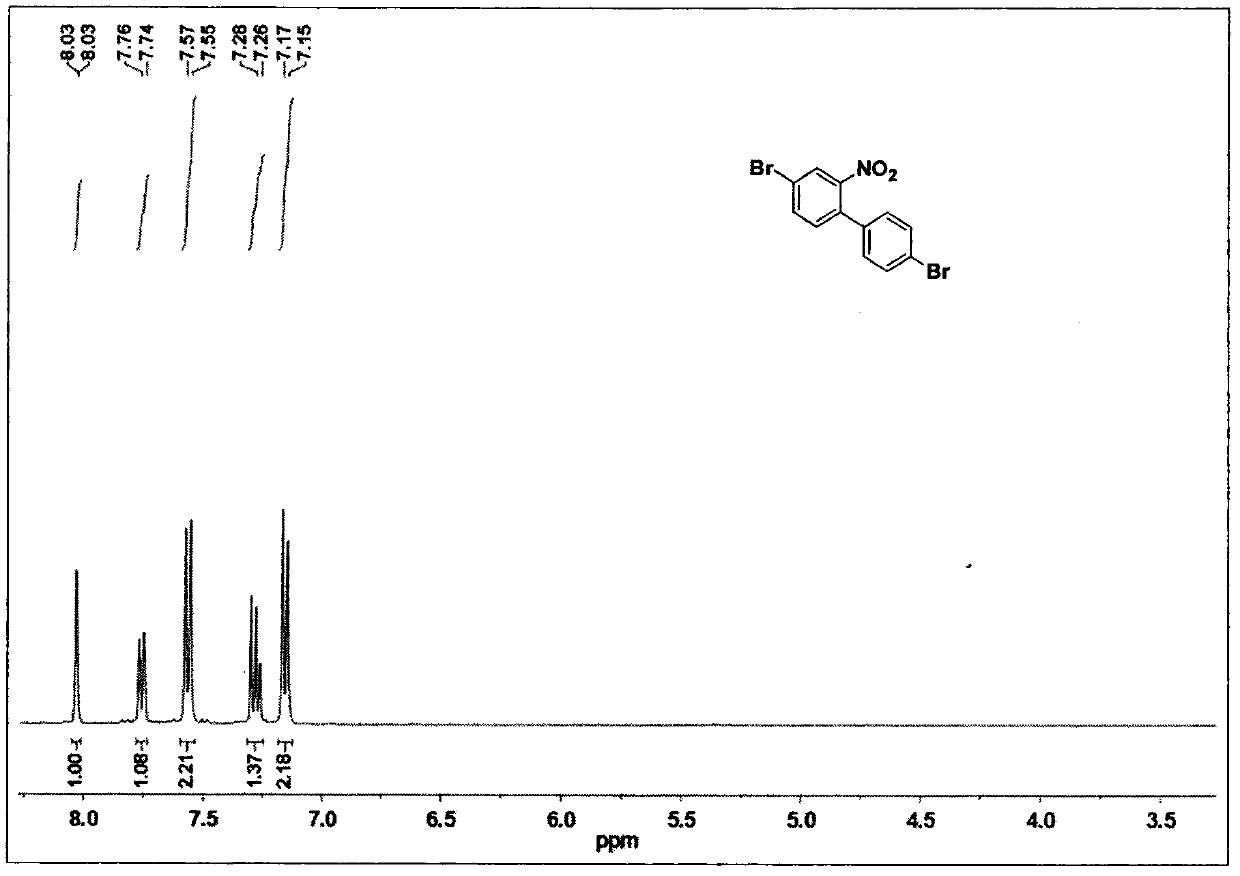
[0040] 4,4'-Dibromo-2-nitro-1,1'-biphenyl (1)
[0041] Dissolve 4,4'-dibromo-1,1'-biphenyl (10.00g, 32.00mmol) in acetic acid (300ml), slowly add HNO at 100°C 3 Mixture with water (7.5ml). After heating at 100°C for 30 minutes, the initially formed precipitate dissolved. After the reaction, the solution was cooled, and a yellow powder was obtained after filtration. Recrystallization in ethanol gave the title product (9.30 g, 81%). 1 H NMR (400MHz, CDCl 3 ): δ8.03(d, J=1.3Hz, 1H), 7.75(m, 1H), 7.56(d, J=7.9Hz, 2H), 7.28(m, 1H), 7.16(d, J=7.9Hz , 2H).
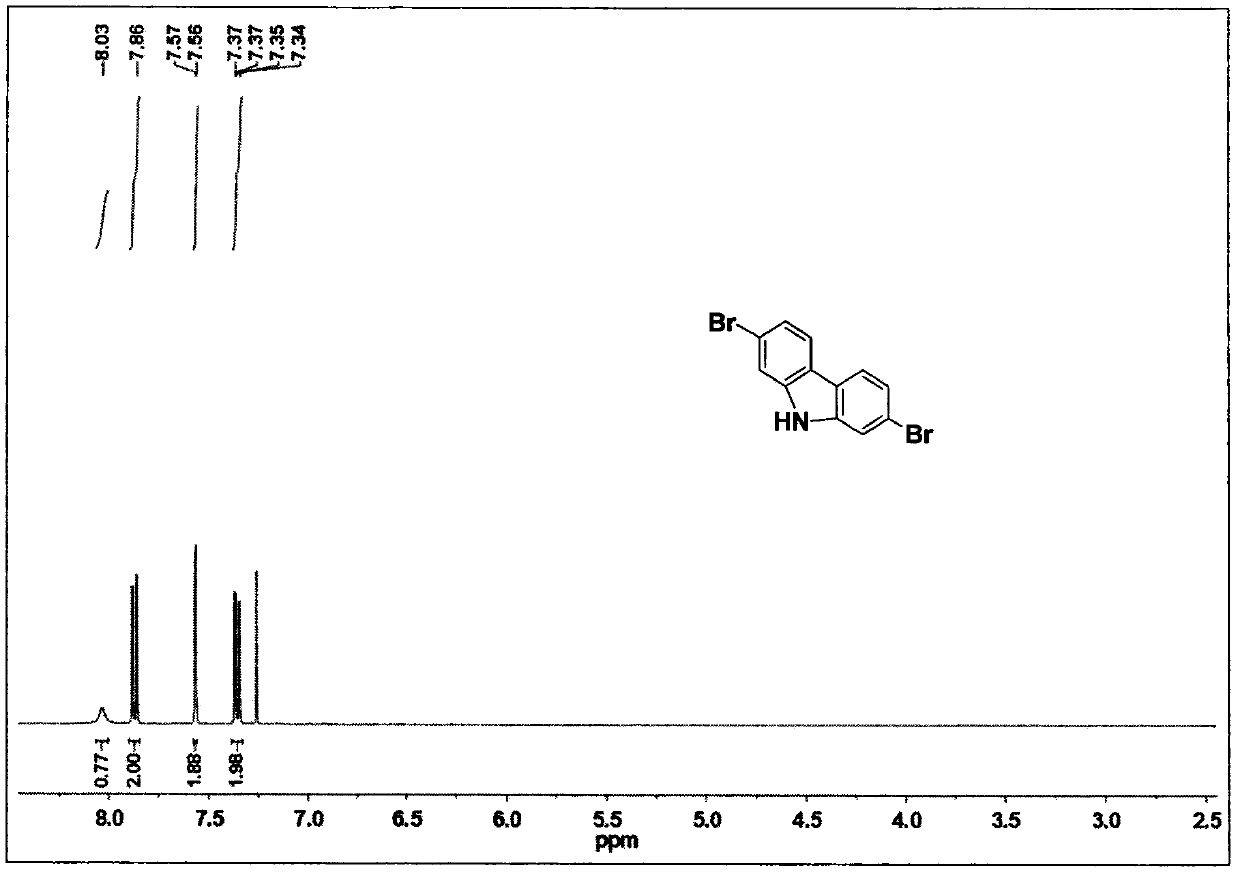
[0042] 2,7-Dibromo-9H-carbazole (2)
[0043] 1 (10.00g, 28.00mmol) and triethyl phosphate (50ml) was heated to reflux for 18 hours, the excess triethyl phosphate was distilled off, and the product was purified by column chromatography (eluent: ethyl acetate: n-hexane = 5:95), the target product (4.66 g, 87%) was obtained. 1 H NMR (400MHz, CDCl 3 ): δ8.03 (s, 1H), 7.87 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com