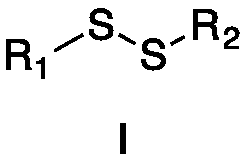
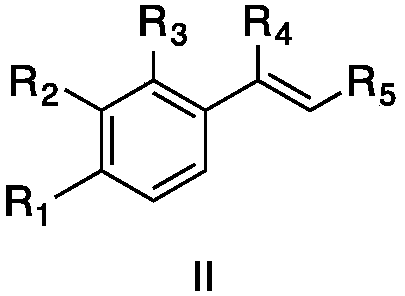
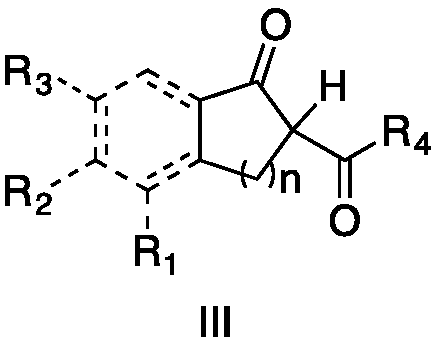
Method for preparing alpha-hydroxymethyl-beta-dicarbonyl compound under catalysis of disulfide excited by visible light
A technology for the preparation of dicarbonyl compounds and catalysis, which is applied in the preparation of organic compounds, chemical instruments and methods, and the formation/introduction of hydroxyl groups. It can solve the problems of narrow substrate types and achieve simple operation, low cost, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 5-chloro-2,3-dihydro-2-hydroxymethyl-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula VI, wherein R 1 , R 3 for H, R 2 for Cl, R 4 for OMe)
[0032] Add 5-chloro-2,3-dihydro-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula II, wherein R1, R3 are H, R2 is Cl, R4 is OMe) in 4 mL of acetonitrile solution , 0.0224g, 0.1mmol), diphenyl disulfide (0.0011g, 0.005mmol) and styrene (0.0156g, 0.15mmol), 10W blue light irradiation for 8 hours to generate 5-chloro-2,3-dihydro-2 -Methyl hydroxymethyl-1-oxo-1H-indene-2-carboxylate, yield 91%. 1 H NMR (400MHz, Chloroform-d) δ7.70 (d, J = 8.2Hz, 1H), 7.51 (s, 1H), 7.41–7.37 (m, 1H), 4.12 (d, J = 11.2Hz, 1H) ,3.90(d,J=11.2Hz,1H),3.71(s,3H),3.54(d,J=17.5Hz,1H),3.39(d,J=17.5Hz,1H),2.53(s,1H) .
Embodiment 2
[0033] Example 2 Preparation of 5-bromo-2,3-dihydro-2-hydroxymethyl-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula VI, wherein R 1 , R 3 for H, R 2 for Br, R 4 for OMe)
[0034]Add 5-bromo-2,3-dihydro-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula III, where R 1 ,R 3 for H, R 2 for Br, R 4 Me, 0.0269g, 0.1mmol), diphenyl disulfide (0.0011g, 0.005mmol) and styrene (0.0156g, 0.15mmol), 10W blue light irradiation for 8 hours to generate 5-bromo-2,3-dihydro -2-Hydroxymethyl-1-oxo-1H-indene-2-carboxylic acid methyl ester, yield 89%. 1 H NMR (400MHz, Chloroform-d) δ7.63(s, 1H), 7.57(d, J=8.0Hz, 1H), 7.49(d, J=8.0Hz, 1H), 4.06(d, J=11.2Hz ,1H),3.82(d,J=11.2Hz,1H),3.65(s,3H),3.48(d,J=17.7Hz,1H),3.33(d,J=17.7Hz,1H),1.57(s ,1H).
Embodiment 3
[0035] Example 3 Preparation of 5-fluoro-2,3-dihydro-2-hydroxymethyl-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula VI, where R 1 for-F, R 2 R 3 for H, R 4 for OMe)
[0036] Add 5-fluoro-2,3-dihydro-1-oxo-1H-indene-2-carboxylic acid methyl ester (formula III, where R 1 for-F, R 2 R 3 for H, R 4 OMe, 0.0208g, 0.1mmol), diphenyl disulfide (0.0011g, 0.005mmol) and styrene (0.0156g, 0.15mmol), 10W blue light irradiation for 8 hours to generate 5-fluoro-2,3-dihydro -2-Hydroxymethyl-1-oxo-1H-indene-2-carboxylic acid methyl ester, yield 90%. 1 H NMR (400MHz, Chloroform-d) δ7.74 (dd, J = 8.6, 5.2Hz, 1H), 7.13 (d, J = 8.6Hz, 1H), 7.07 (td, J = 8.6, 2.2Hz, 1H) ,4.09(d,J=11.3Hz,1H),3.83(d,J=11.3Hz,1H),3.68(s,2H),3.50(d,J=17.7Hz,1H),3.35(d,J= 17.8Hz, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com